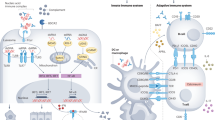
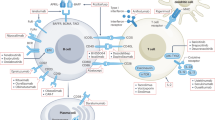
Abstract
Lupus nephritis (LN) is a form of glomerulonephritis that constitutes one of the most severe organ manifestations of the autoimmune disease systemic lupus erythematosus (SLE). Most patients with SLE who develop LN do so within 5 years of an SLE diagnosis and, in many cases, LN is the presenting manifestation resulting in the diagnosis of SLE. Understanding of the genetic and pathogenetic basis of LN has improved substantially over the past few decades. Treatment of LN usually involves immunosuppressive therapy, typically with mycophenolate mofetil or cyclophosphamide and with glucocorticoids, although these treatments are not uniformly effective. Despite increased knowledge of disease pathogenesis and improved treatment options, LN remains a substantial cause of morbidity and death among patients with SLE. Within 10 years of an initial SLE diagnosis, 5–20% of patients with LN develop end-stage kidney disease, and the multiple comorbidities associated with immunosuppressive treatment, including infections, osteoporosis and cardiovascular and reproductive effects, remain a concern. Clearly, early and accurate diagnosis of LN and prompt initiation of therapy are of vital importance to improve outcomes in patients with SLE.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Singh, S., Saxena, R. & Palmer, B. F. Lupus nephritis. Am. J. Med. Sci. 337, 451–460 (2009).
Pons-Estel, G. J., Serrano, R., Plasín, M. A., Espinosa, G. & Cervera, R. Epidemiology and management of refractory lupus nephritis. Autoimmun. Rev. 10, 655–663 (2011).
Cervera, R. et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine 82, 299–308 (2003).
Croca, S. C., Rodrigues, T. & Isenberg, D. A. Assessment of a lupus nephritis cohort over a 30-year period. Rheumatology 50, 1424–1430 (2011). An outcome analysis of 156 patients with LN, around 50 from each decade, indicating the trends in mortality, ESKD rate and prevalence of other features over time in a European centre.
Bernatsky, S. et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 54, 2550–2557 (2006).
Moroni, G. et al. Changing patterns in clinical-histological presentation and renal outcome over the last five decades in a cohort of 499 patients with lupus nephritis. Ann. Rheum. Dis. 77, 1318–1325 (2018).
Houssiau, F. A. Management of lupus nephritis: an update. J. Am. Soc. Nephrol. 15, 2694–2704 (2004).
Houssiau, F. A. et al. Early response to immunosuppressive therapy predicts good renal outcome in lupus nephritis: lessons from long-term followup of patients in the Euro-Lupus Nephritis Trial. Arthritis Rheum. 50, 3934–3940 (2004).
Houssiau, F. & Ginzler, E. Current treatment of lupus nephritis. Lupus 17, 426–430 (2008).
Borchers, A. T., Naguwa, S. M., Shoenfeld, Y. & Gershwin, M. E. The geoepidemiology of systemic lupus erythematosus. Autoimmun. Rev. 9, A277–A287 (2010).
Stojan, G. & Petri, M. Epidemiology of systemic lupus erythematosus: an update. Curr. Opin. Rheumatol. 30, 144–150 (2018).
Rees, F., Doherty, M., Grainge, M. J., Lanyon, P. & Zhang, W. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology 56, 1945–1961 (2017).
Cervera, R. et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. Medicine 72, 113–124 (1993).
Kasitanon, N., Magder, L. S. & Petri, M. Predictors of survival in systemic lupus erythematosus. Medicine 85, 147–156 (2006).
To, C. H. & Petri, M. Is antibody clustering predictive of clinical subsets and damage in systemic lupus erythematosus? Arthritis Rheum. 52, 4003–4010 (2005).
Seligman, V. A., Lum, R. F., Olson, J. L., Li, H. & Criswell, L. A. Demographic differences in the development of lupus nephritis: a retrospective analysis. Am. J. Med. 112, 726–729 (2002).
Chang, D.-M., Chang, C.-C., Kuo, S.-Y., Chu, S.-J. & Chang, M.-L. The clinical features and prognosis of male lupus in Taiwan. Lupus 7, 462–468 (1998).
Mok, C. C., Lau, C. S., Chan, T. M. & Wong, R. W. Clinical characteristics and outcome of southern Chinese males with systemic lupus erythematosus. Lupus 8, 188–196 (1999).
Garcia, M. A. et al. Male systemic lupus erythematosus in a Latin-American inception cohort of 1214 patients. Lupus 14, 938–946 (2005).
Andrade, R. M. et al. Accelerated damage accrual among men with systemic lupus erythematosus: XLIV. Results from a multiethnic US cohort. Arthritis Rheum. 56, 622–630 (2007).
Stefanidou, S. et al. Clinical expression and morbidity of systemic lupus erythematosus during a post-diagnostic 5-year follow-up: a male:female comparison. Lupus 20, 1090–1094 (2011).
Carreño, L. et al. Immunological and clinical differences between juvenile and adult onset of systemic lupus erythematosus. Lupus 8, 287–292 (1999).
Bader-Meunier, B. et al. Initial presentation of childhood-onset systemic lupus erythematosus: a French multicenter study. J. Pediatr. 146, 648–653 (2005).
Ramírez Gómez, L. et al. Childhood systemic lupus erythematosus in Latin America. The GLADEL experience in 230 children. Lupus 17, 596–604 (2008).
Hoffman, I. E. A. et al. Juvenile-onset systemic lupus erythematosus: different clinical and serological pattern than adult-onset systemic lupus erythematosus. Ann. Rheum. Dis. 68, 412–415 (2009).
Bastian, H. M. et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus 11, 152–160 (2002).
Feldman, C. H. et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum. 65, 753–763 (2013).
Jakes, R. W. et al. Systematic review of the epidemiology of systemic lupus erythematosus in the Asia-Pacific region: prevalence, incidence, clinical features, and mortality. Arthritis Care Res. 64, 159–168 (2012).
Hanly, J. G. et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology 55, 252–262 (2016). This multiethnic inception cohort study indicates that LN occurred in 38.3% of patients with SLE and was associated with risk of ESKD and death and lower HRQOL.
Maningding, E., Dall’Era, M., Trupin, L., Murphy, L. B. & Yazdany, J. Racial/ethnic differences in prevalence of and time to onset of SLE manifestations: the California lupus surveillance project (CLSP). Arthritis Care Res. https://doi.org/10.1002/acr.23887 (2019). This epidemiological study demonstrates that black, Asian/Pacific Islander and Hispanic individuals are at increased risk of developing severe LN following SLE diagnosis.
Kuo, C.-F. et al. Familial aggregation of systemic lupus erythematosus and coaggregation of autoimmune diseases in affected families. JAMA Intern. Med. 175, 1518–1526 (2015).
Mohan, C. & Putterman, C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat. Rev. Nephrol. 11, 329–341 (2015).
Deng, Y. & Tsao, B. P. Updates in lupus genetics. Curr. Rheumatol. Rep. 19, 68 (2017).
Lo, M. S. Monogenic lupus. Curr. Rheumatol. Rep. 18, 71 (2016).
Goulielmos, G. N. et al. The genetics and molecular pathogenesis of systemic lupus erythematosus (SLE) in populations of different ancestry. Gene 668, 59–72 (2018).
Imgenberg-Kreuz, J. et al. DNA methylation mapping identifies gene regulatory effects in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 77, 736–743 (2018).
Souyris, M. et al. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 3, eaap8855 (2018). This landmark article provides evidence that escape of X chromosome genes from X inactivation can lead to immune activation, and this may explain, in part, the higher prevalence of autoimmunity in females.
Parks, C. G., de Souza Espindola Santos, A., Barbhaiya, M. & Costenbader, K. H. Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best. Pract. Res. Clin. Rheumatol. 31, 306–320 (2017).
Barbhaiya, M. et al. Cigarette smoking and the risk of systemic lupus erythematosus, overall and by anti-double stranded DNA antibody subtype, in the nurses’ health study cohorts. Ann. Rheum. Dis. 77, 196–202 (2018).
Petri, M. et al. Combined oral contraceptives in women with systemic lupus erythematosus. N. Engl. J. Med. 353, 2550–2558 (2005).
Andreoli, L. et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann. Rheum. Dis. 76, 476–485 (2017).
Parks, C. & De Roos, A. Pesticides, chemical and industrial exposures in relation to systemic lupus erythematosus. Lupus 23, 527–536 (2014).
Barbhaiya, M. & Costenbader, K. H. Environmental exposures and the development of systemic lupus erythematosus. Curr. Opin. Rheumatol. 28, 497–505 (2016).
Barbhaiya, M. & Costenbader, K. Ultraviolet radiation and systemic lupus erythematosus. Lupus 23, 588–595 (2014).
Wang, B., Shao, X., Wang, D., Xu, D. & Zhang, J.-A. Vaccinations and risk of systemic lupus erythematosus and rheumatoid arthritis: a systematic review and meta-analysis. Autoimmun. Rev. 16, 756–765 (2017).
Abdul Kadir, W. D., Jamil, A., Shaharir, S. S., Md Nor, N. & Abdul Gafor, A. H. Photoprotection awareness and practices among patients with systemic lupus erythematosus and its association with disease activity and severity. Lupus 27, 1287–1295 (2018).
Silverman, G. J. The microbiome in SLE pathogenesis. Nat. Rev Rheumatol. 15, 72–74 (2019). This is a comprehensive review of microbiome changes in SLE, with a thoughtful discussion of how they may influence disease.
Hevia, A. et al. Intestinal dysbiosis associated with systemic lupus erythematosus. mBio 5, e01548-14 (2014).
van der Meulen, T. A. et al. Shared gut, but distinct oral microbiota composition in primary Sjögren’s syndrome and systemic lupus erythematosus. J. Autoimmun. 97, 77–87 (2019).
He, Z., Shao, T., Li, H., Xie, Z. & Wen, C. Alterations of the gut microbiome in Chinese patients with systemic lupus erythematosus. Gut Pathog. 8, 64 (2016).
Lyn-Cook, B. D. et al. Increased expression of Toll-like receptors (TLRs) 7 and 9 and other cytokines in systemic lupus erythematosus (SLE) patients: ethnic differences and potential new targets for therapeutic drugs. Mol. Immunol. 61, 38–43 (2014).
Lee, Y. H., Choi, S. J., Ji, J. D. & Song, G. G. Association between Toll-like receptor polymorphisms and systemic lupus erythematosus: a meta-analysis update. Lupus 25, 593–601 (2016).
Devarapu, S. K. & Anders, H.-J. Toll-like receptors in lupus nephritis. J. Biomed. Sci. 25, 35 (2018).
Sharma, S., Fitzgerald, K. A., Cancro, M. P. & Marshak-Rothstein, A. Nucleic acid-sensing receptors: rheostats of autoimmunity and autoinflammation. J. Immunol. 195, 3507–3512 (2015).
Wang, J. et al. Association of abnormal elevations in IFIT3 with overactive cyclic GMP-AMP synthase/stimulator of interferon genes signaling in human systemic lupus erythematosus monocytes. Arthritis Rheumatol. 70, 2036–2045 (2018).
Kato, Y. et al. Apoptosis-derived membrane vesicles drive the cGAS–STING pathway and enhance type I IFN production in systemic lupus erythematosus. Ann. Rheum. Dis. 77, 1507–1515 (2018).
Salvi, V. et al. Exosome-delivered microRNAs promote IFN-α secretion by human plasmacytoid DCs via TLR7. JCI Insight 3, e98204 (2018).
Dieker, J. et al. Circulating apoptotic microparticles in systemic lupus erythematosus patients drive the activation of dendritic cell subsets and prime neutrophils for NETosis: proinflammatory effects of circulating microparticles in SLE. Arthritis Rheumatol. 68, 462–472 (2016).
Sisirak, V. et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell 166, 88–101 (2016).
Caielli, S. et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J. Exp. Med. 213, 697–713 (2016). This article ties together the pathogenetic roles of oxidized mitochondrial DNA and type I interferon in SLE.
Gkirtzimanaki, K. et al. IFNα impairs autophagic degradation of mtDNA promoting autoreactivity of SLE monocytes in a STING-dependent fashion. Cell Rep. 25, 921–933.e5 (2018).
Elkon, K. B. Review: cell death, nucleic acids, and immunity: inflammation beyond the grave. Arthritis Rheumatol. 70, 805–816 (2018). This review provides a critical discussion of how aberrant nucleic acid sensing may relate to the pathogenesis of SLE.
Banchereau, R. et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell 165, 1548–1550 (2016).
Jourde-Chiche, N. et al. Modular transcriptional repertoire analyses identify a blood neutrophil signature as a candidate biomarker for lupus nephritis. Rheumatology 56, 477–487 (2017).
Rother, N. & van der Vlag, J. Disturbed T cell signaling and altered Th17 and regulatory T cell subsets in the pathogenesis of systemic lupus erythematosus. Front. Immunol. 6, 610 (2015).
Kim, S. J., Lee, K. & Diamond, B. Follicular helper T cells in systemic lupus erythematosus. Front. Immunol. 9, 1793 (2018).
Zharkova, O. et al. Pathways leading to an immunological disease: systemic lupus erythematosus. Rheumatology 56, i55–i66 (2017).
Liu, C.-C., Manzi, S. & Ahearn, J. M. in Dubois’ Lupus Erythematosus and Related Syndromes (eds Wallace, D. J. & Hahn, B. H.) 152–165 (Elsevier, 2013).
Bao, L., Cunningham, P. N. & Quigg, R. J. Complement in lupus nephritis: new perspectives. Kidney Dis. 1, 91–99 (2015).
Ho, A., Barr, S. G., Magder, L. S. & Petri, M. A decrease in complement is associated with increased renal and hematologic activity in patients with systemic lupus erythematosus. Arthritis Rheum. 44, 2350–2357 (2001).
Gandino, I. J. et al. Complement levels and risk of organ involvement in patients with systemic lupus erythematosus. Lupus Sci. Med. 4, e000209 (2017).
Swaak, A. J., Groenwold, J. & Bronsveld, W. Predictive value of complement profiles and anti-dsDNA in systemic lupus erythematosus. Ann. Rheumatic Dis. 45, 359–366 (1986).
Sturfelt, G. & Truedsson, L. Complement and its breakdown products in SLE. Rheumatology 44, 1227–1232 (2005).
Senaldi, G., Makinde, V. A., Vergani, D. & Isenberg, D. A. Correlation of the activation of the fourth component of complement (C4) with disease activity in systemic lupus erythematosus. Ann. Rheum. Dis. 47, 913–917 (1988).
Song, D. et al. Complement alternative pathway’s activation in patients with lupus nephritis. Am. J. Med. Sci. 353, 247–257 (2017).
Liu, Y. & Anders, H.-J. Lupus nephritis: from pathogenesis to targets for biologic treatment. Nephron Clin. Pract. 128, 224–231 (2014).
Yu, F., Haas, M., Glassock, R. & Zhao, M.-H. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat. Rev. Nephrol. 13, 483–495 (2017).
Kaul, A. et al. Systemic lupus erythematosus. Nat. Rev. Dis. Primers 2, 16039 (2016).
Anders, H.-J. Nephropathic autoantigens in the spectrum of lupus nephritis. Nat. Rev. Nephrol. 15, 595–596 (2019).
Doria, A. & Gatto, M. Nephritogenic-antinephritogenic antibody network in lupus glomerulonephritis. Lupus 21, 1492–1496 (2012).
Bruschi, M. et al. Glomerular autoimmune multicomponents of human lupus nephritis in vivo (2): planted antigens. J. Am. Soc. Nephrol. 26, 1905–1924 (2015).
Bassi, N. et al. PTX3, anti-PTX3, and anti-C1q autoantibodies in lupus glomerulonephritis. Clin. Rev. Allergy Immunol. 49, 217–226 (2015).
Migliorini, A. & Anders, H.-J. A novel pathogenetic concept — antiviral immunity in lupus nephritis. Nat. Rev. Nephrol. 8, 183–189 (2012).
Sciascia, S., Cuadrado, M. J., Khamashta, M. & Roccatello, D. Renal involvement in antiphospholipid syndrome. Nat. Rev. Nephrol. 10, 279–289 (2014).
Turner-Stokes, T. et al. Positive antineutrophil cytoplasmic antibody serology in patients with lupus nephritis is associated with distinct histopathologic features on renal biopsy. Kidney Int. 92, 1223–1231 (2017).
Ryu, M. et al. Plasma leakage through glomerular basement membrane ruptures triggers the proliferation of parietal epithelial cells and crescent formation in non-inflammatory glomerular injury: crescents without glomerular inflammation. J. Pathol. 228, 482–494 (2012).
Sethi, S. et al. Exostosin 1/exostosin 2-associated membranous nephropathy. J. Am. Soc. Nephrol. 30, 1123–1136 (2019). The first description of a novel tissue biomarker of membranous nephropathy related to autoimmune diseases such as LN.
Devarapu, S. K., Lorenz, G., Kulkarni, O. P., Anders, H.-J. & Mulay, S. R. Cellular and molecular mechanisms of autoimmunity and lupus nephritis. Int. Rev. Cell Mol. Biol. 332, 43–154 (2017).
Hiepe, F. et al. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat. Rev. Rheumatol. 7, 170–178 (2011). This article reviews the profound role that long-lived plasma cells may have in LN, and highlights their potential as therapeutic targets.
Hiepe, F. & Radbruch, A. Plasma cells as an innovative target in autoimmune disease with renal manifestations. Nat. Rev. Nephrol. 12, 232–240 (2016).
Huang, X. et al. Autologous hematopoietic stem cell transplantation for refractory lupus nephritis. Clin. J. Am. Soc. Nephrol. 14, 719–727 (2019).
Alexander, T. et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann. Rheum. Dis. 74, 1474–1478 (2015).
Sun, C. et al. Safety and efficacy of targeting CD138 with a chimeric antigen receptor for the treatment of multiple myeloma. Oncotarget 10, 2369–2383 (2019).
Arazi, A. et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 20, 902–914 (2019).
Kang, S. et al. BAFF induces tertiary lymphoid structures and positions T cells within the glomeruli during lupus nephritis. J. Immunol. 198, 2602–2611 (2017).
Der, E. et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat. Immunol. 20, 915–927 (2019).
Bethunaickan, R. et al. Identification of stage-specific genes associated with lupus nephritis and response to remission induction in (NZB × NZW)F1 and NZM2410 mice. Arthritis Rheumatol. 66, 2246–2258 (2014).
Kidney disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3, 1–150 (2013). The current global guideline for evaluation and management of CKD irrespective of its cause.
Low Birth Weight and Nephron Number Working Group. The impact of kidney development on the life course: a consensus document for action. Nephron 136, 3–49 (2017).
Romagnani, P. et al. Chronic kidney disease. Nat. Rev. Dis. Primers 3, 17088 (2017).
Mackay, M. et al. Establishing surrogate kidney end points for lupus nephritis clinical trials: development and validation of a novel approach to predict future kidney outcomes. Arthritis Rheumatol. 71, 411–419 (2019). An analysis of multiple trial data sets presenting a proteinuria threshold that is associated with fortunate long-term outcomes.
Mageau, A. et al. The burden of chronic kidney disease in systemic lupus erythematosus: a nationwide epidemiologic study. Autoimmun. Rev. 18, 733–737 (2019).
Mejía-Vilet, J. M. et al. Renal flare prediction and prognosis in lupus nephritis Hispanic patients. Lupus 25, 315–324 (2016).
Momtaz, M. et al. Retrospective analysis of nephritis response and renal outcome in a cohort of 928 Egyptian lupus nephritis patients: a university hospital experience. Lupus 26, 1564–1570 (2017).
Genovese, G. et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329, 841–845 (2010).
Tzur, S. et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum. Genet. 128, 345–350 (2010).
Freedman, B. I. et al. End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol. 66, 390–396 (2014). A study that establishes the diagnostic value of APOL1 variants in predicting the progression of CKD in patients with LN.
Lin, C. P. et al. Role of MYH9 and APOL1 in African and non-African populations with lupus nephritis. Genes Immun. 13, 232–238 (2012).
Romagnani, P. et al. Next generation sequencing and functional analysis of patient urine renal progenitor-derived podocytes to unravel the diagnosis underlying refractory lupus nephritis. Nephrol. Dial. Transplant. 31, 1541–1545 (2016).
Mok, C.-C. Understanding lupus nephritis: diagnosis, management, and treatment options. Int. J. Womens Health 4, 213–222 (2012).
Tan, E. M. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus: revised criteria for SLE. Arthritis Rheum. 25, 1271–1277 (1982).
Petri, M. et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64, 2677–2686 (2012).
Aringer, M. et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann. Rheum. Dis. 78, 1151–1159 (2019).
Fine, D. M. et al. A prospective study of protein excretion using short-interval timed urine collections in patients with lupus nephritis. Kidney Int. 76, 1284–1288 (2009).
Font, J. et al. Silent renal disease in systemic lupus erythematosus. Clin. Nephrol. 27, 283–288 (1987).
Gordon, C. et al. European consensus statement on the terminology used in the management of lupus glomerulonephritis. Lupus 18, 257–263 (2009). This article provides guidance regarding LN classification and definitions of induction, response, flare and maintenance.
Bertsias, G. K. et al. Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann. Rheum. Dis. 71, 1771–1782 (2012). This article provides EULAR/ERA–EDTA treatment recommendations for the management of LN. An update is in preparation.
Hahn, B. H. et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. 64, 797–808 (2012). This article provides ACR treatment recommendations for the management of LN.
Bihl, G. R., Petri, M. & Fine, D. M. Kidney biopsy in lupus nephritis: look before you leap. Nephrol. Dial. Transplant. 21, 1749–1752 (2006).
Tamirou, F. et al. A proteinuria cut-off level of 0.7 g/day after 12 months of treatment best predicts long-term renal outcome in lupus nephritis: data from the MAINTAIN nephritis trial. Lupus Sci. Med. 2, e000123 (2015).
Ugolini-Lopes, M. R. et al. Early proteinuria response: a valid real-life situation predictor of long-term lupus renal outcome in an ethnically diverse group with severe biopsy-proven nephritis? Lupus Sci. Med 4, e000213 (2017).
Dall’Era, M. et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-Lupus nephritis cohort. Arthritis Rheumatol. 67, 1305–1313 (2015).
Zickert, A., Sundelin, B., Svenungsson, E. & Gunnarsson, I. Role of early repeated renal biopsies in lupus nephritis. Lupus Sci. Med. 1, e000018 (2014).
Malvar, A. et al. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol. Dial. Transplant. 32, 1338–1344 (2017).
Parikh, S. V., Nagaraja, H. N., Hebert, L. & Rovin, B. H. Renal flare as a predictor of incident and progressive CKD in patients with lupus nephritis. Clin. J. Am. Soc. Nephrol. 9, 279–284 (2014).
Ioannidis, J. P. et al. Remission, relapse, and re-remission of proliferative lupus nephritis treated with cyclophosphamide. Kidney Int. 57, 258–264 (2000).
De Rosa, M. et al. A prospective observational cohort study highlights kidney biopsy findings of lupus nephritis patients in remission who flare following withdrawal of maintenance therapy. Kidney Int. 94, 788–794 (2018). Zickert et al. (2014), Malvar et al. (2017) and De Rosa et al. (2018) demonstrate discordance between clinical outcome and histological findings following immunosuppressive treatment of LN.
Appel, G. B., Silva, F. G., Pirani, C. L., Meltzer, J. I. & Estes, D. Renal involvement in systemic lupus erythematosus (SLE): a study of 56 patients emphasizing histologic classification. Medicine 57, 371–410 (1978).
Weening, J. J. et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 65, 521–530 (2004).
Churg, J., Bernstein, J. & Glassock, R. J. Reviews and notes: nephrology: renal disease: classification and atlas of glomerular diseases. Ann. Intern. Med. 123, 80–80 (1995).
Pirani, C. L., Pollak, V. E. & Schwartz, F. D. The reproducibility of semiquantitative analyses of renal histology. Nephron 1, 230–237 (1964).
Morel-Maroger, L. et al. The course of lupus nephritis: contribution of serial renal biopsies. Adv. Nephrol. Necker Hosp. 6, 79–118 (1976).
Austin, H. A. et al. Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am. J. Med. 75, 382–391 (1983).
Schwartz, M. M. et al. Irreproducibility of the activity and chronicity indices limits their utility in the management of lupus nephritis. Am. J. Kidney Dis. 21, 374–377 (1993).
Contreras, G. et al. Factors associated with poor outcomes in patients with lupus nephritis. Lupus 14, 890–895 (2005).
Bajema, I. M. et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 93, 789–796 (2018).
Miyakis, S. et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 4, 295–306 (2006).
Daugas, E. et al. Antiphospholipid syndrome nephropathy in systemic lupus erythematosus. J. Am. Soc. Nephrol. 13, 42–52 (2002).
Tektonidou, M. G., Sotsiou, F., Nakopoulou, L., Vlachoyiannopoulos, P. G. & Moutsopoulos, H. M. Antiphospholipid syndrome nephropathy in patients with systemic lupus erythematosus and antiphospholipid antibodies: prevalence, clinical associations, and long-term outcome. Arthritis Rheum. 50, 2569–2579 (2004).
Gerhardsson, J. et al. Histological antiphospholipid-associated nephropathy versus lupus nephritis in patients with systemic lupus erythematosus: an observational cross-sectional study with longitudinal follow-up. Arthritis Res. Ther. 17, 109 (2015).
Song, D. et al. The spectrum of renal thrombotic microangiopathy in lupus nephritis. Arthritis Res. Ther. 15, R12 (2013).
Zheng, H. et al. Antiphospholipid antibody profiles in lupus nephritis with glomerular microthrombosis: a prospective study of 124 cases. Arthritis Res. Ther. 11, R93 (2009).
Jordan, N. et al. Association of thrombotic microangiopathy and intimal hyperplasia with bleeding post-renal biopsy in antiphospholipid antibody-positive patients. Arthritis Care Res. 66, 725–731 (2014).
Nielly, H. et al. Safety and effectiveness of transjugular renal biopsy for systemic lupus erythematosus and antiphospholipid antibody syndrome patients taking antithrombotics. Nephrol. Dial. Transplant. https://doi.org/10.1093/ndt/gfz085 (2019).
Moroni, G. et al. Antiphospholipid antibodies are associated with an increased risk for chronic renal insufficiency in patients with lupus nephritis. Am. J. Kidney Dis. 43, 28–36 (2004).
Frampton, G., Hicks, J. & Cameron, J. S. Significance of anti-phospholipid antibodies in patients with lupus nephritis. Kidney Int. 39, 1225–1231 (1991).
Mehrani, T. & Petri, M. IgM anti-ß2 glycoprotein I is protective against lupus nephritis and renal damage in systemic lupus erythematosus. J. Rheumatol. 38, 450–453 (2011).
Bhandari, S. Association of anticardiolipin antibodies with intraglomerular thrombi and renal dysfunction in lupus nephritis. QJM 91, 401–409 (1998).
Stratta, P. et al. Catastrophic antiphospholipid syndromes in systemic lupus erythematosus. Ren. Fail. 21, 49–61 (1999).
Parodis, I. et al. Antiphospholipid antibodies in lupus nephritis. PLOS ONE 11, e0158076 (2016).
Soliman, S. & Mohan, C. Lupus nephritis biomarkers. Clin. Immunol. 185, 10–20 (2017).
Moroni, G. et al. The value of a panel of autoantibodies for predicting the activity of lupus nephritis at time of renal biopsy. J. Immunol. Res. 2015, 106904 (2015).
Olson, S. W. et al. Elevated subclinical double-stranded DNA antibodies and future proliferative lupus nephritis. Clin. J. Am. Soc. Nephrol. 8, 1702–1708 (2013).
Wakiguchi, H., Takei, S., Kubota, T., Miyazono, A. & Kawano, Y. Treatable renal disease in children with silent lupus nephritis detected by baseline biopsy: association with serum C3 levels. Clin. Rheumatol. 36, 433–437 (2017).
Kwon, O. C. et al. Predicting eventual development of lupus nephritis at the time of diagnosis of systemic lupus erythematosus. Semin. Arthritis Rheum. 48, 462–466 (2018).
Buyon, J. P. et al. Kidney outcomes and risk factors for nephritis (flare/de novo) in a multiethnic cohort of pregnant patients with lupus. Clin. J. Am. Soc. Nephrol. 12, 940–946 (2017).
Marto, N. Anti-C1q antibodies in nephritis: correlation between titres and renal disease activity and positive predictive value in systemic lupus erythematosus. Ann. Rheum. Dis. 64, 444–448 (2004).
Moroni, G. et al. Are laboratory tests useful for monitoring the activity of lupus nephritis? A 6-year prospective study in a cohort of 228 patients with lupus nephritis. Ann. Rheum. Dis. 68, 234–237 (2009).
Julkunen, H., Ekblom-Kullberg, S. & Miettinen, A. Nonrenal and renal activity of systemic lupus erythematosus: a comparison of two anti-C1q and five anti-dsDNA assays and complement C3 and C4. Rheumatol. Int. 32, 2445–2451 (2012).
Yang, X.-W., Tan, Y., Yu, F. & Zhao, M.-H. Combination of anti-C1q and anti-dsDNA antibodies is associated with higher renal disease activity and predicts renal prognosis of patients with lupus nephritis. Nephrol. Dial. Transplant. 27, 3552–3559 (2012).
Orbai, A.-M. et al. Anti-C1q antibodies in systemic lupus erythematosus. Lupus 24, 42–49 (2015).
Fang, Q.-Y. et al. Anti-C1q antibodies and IgG subclass distribution in sera from Chinese patients with lupus nephritis. Nephrol. Dial. Transplant. 24, 172–178 (2008).
Tan, Y. et al. Detection of anti-C1q antibodies and anti-C1q globular head domain antibodies in sera from Chinese patients with lupus nephritis. Mol. Immunol. 46, 2178–2182 (2009).
Pang, Y. et al. Serum A08 C1q antibodies are associated with disease activity and prognosis in Chinese patients with lupus nephritis. Kidney Int. 90, 1357–1367 (2016). This study reports that the serum level of anti-C1q A08 antibodies correlated better with LN relapse than that of antibodies to other forms of C1q.
Katsumata, Y. et al. Anti-C1q antibodies are associated with systemic lupus erythematosus global activity but not specifically with nephritis: a controlled study of 126 consecutive patients. Arthritis Rheum. 63, 2436–2444 (2011).
Tan, Y. et al. Autoantibodies against monomeric C-reactive protein in sera from patients with lupus nephritis are associated with disease activity and renal tubulointerstitial lesions. Hum. Immunol. 69, 840–844 (2008).
Sjöwall, C., Zickert, A., Skogh, T., Wetterö, J. & Gunnarsson, I. Serum levels of autoantibodies against C-reactive protein correlate with renal disease activity and response to therapy in lupus nephritis. Arthritis Res. Ther. 11, R188 (2009).
Li, Q.-y et al. Autoantibodies against C-reactive protein influence complement activation and clinical course in lupus nephritis. J. Am. Soc. Nephrol. 28, 3044–3054 (2017). This study provides a mechanistic link between autoantibody binding to modified C-reactive protein and complement factor H activity in LN.
Simon, J. A. Anti-nucleosome antibodies in patients with systemic lupus erythematosus of recent onset. Potential utility as a diagnostic tool and disease activity marker. Rheumatology 43, 220–224 (2003).
Manson, J. J. et al. Relationship between anti-dsDNA, anti-nucleosome and anti-alpha-actinin antibodies and markers of renal disease in patients with lupus nephritis: a prospective longitudinal study. Arthritis Res. Ther. 11, R154 (2009).
Vikerfors, A. et al. Clinical manifestations and anti-phospholipid antibodies in 712 patients with systemic lupus erythematosus: evaluation of two diagnostic assays. Rheumatology 52, 501–509 (2013).
Illei, G. G., Tackey, E., Lapteva, L. & Lipsky, P. E. Biomarkers in systemic lupus erythematosus: I. General overview of biomarkers and their applicability. Arthritis Rheum. 50, 1709–1720 (2004).
Rovin, B. H. et al. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J. Am. Soc. Nephrol. 16, 467–473 (2005).
Qi, S., Chen, Q., Xu, D., Xie, N. & Dai, Y. Clinical application of protein biomarkers in lupus erythematosus and lupus nephritis. Lupus 27, 1582–1590 (2018).
Zhang, X. et al. A composite urine biomarker reflects interstitial inflammation in lupus nephritis kidney biopsies. Kidney Int. 81, 401–406 (2012).
Faurschou, M., Starklint, H., Halberg, P. & Jacobsen, S. Prognostic factors in lupus nephritis: diagnostic and therapeutic delay increases the risk of terminal renal failure. J. Rheumatol. 33, 1563–1569 (2006).
Donadio, J. V., Holley, K. E., Ferguson, R. H. & Ilstrup, D. M. Treatment of diffuse proliferative lupus nephritis with prednisone and combined prednisone and cyclophosphamide. N. Engl. J. Med. 299, 1151–1155 (1978).
Austin, H. A. 3rd et al. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N. Engl. J. Med. 314, 614–619 (1986). This landmark clinical trial from the NIH establishes intravenous cyclophosphamide therapy as the gold standard in the treatment of LN.
Bono, L. The very long-term prognosis and complications of lupus nephritis and its treatment. QJM 92, 211–218 (1999).
Houssiau, F. A. et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide: immunosuppressive therapy in lupus nephritis. Arthritis Rheum. 46, 2121–2131 (2002). A randomized clinical trial that shows efficacy and safety of low-dose intravenous cyclophosphamide therapy (ELNT regimen) in the treatment of LN.
ACCESS Trial Group. Treatment of lupus nephritis with abatacept: the abatacept and cyclophosphamide combination efficacy and safety study: abatacept in lupus nephritis. Arthritis Rheumatol. 66, 3096–3104 (2014).
Fanouriakis, A. et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 78, 736–745 (2019).
Chan, T. M. et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. N. Engl. J. Med. 343, 1156–1162 (2000).
Ginzler, E. M. et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N. Engl. J. Med. 353, 2219–2228 (2005). A randomized clinical trial from the USA that shows non-inferiority of MMF compared with intravenously administered cyclophosphamide as an induction regimen in the treatment of LN.
Appel, G. B. et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J. Am. Soc. Nephrol. 20, 1103–1112 (2009). A large global randomized clinical trial (ALMS) that shows efficacy of MMF as an induction agent in the treatment of LN.
Isenberg, D. et al. Influence of race/ethnicity on response to lupus nephritis treatment: the ALMS study. Rheumatology 49, 128–140 (2010).
Soares, P. M. et al. Gonad evaluation in male systemic lupus erythematosus. Arthritis Rheum. 56, 2352–2361 (2007).
Mok, C. C. et al. Overview of lupus nephritis management guidelines and perspective from Asia. Int. J. Rheum. Dis. 16, 625–636 (2013).
Zahr, N. et al. Mycophenolic acid area under the curve correlates with disease activity in lupus patients treated with mycophenolate mofetil. Arthritis Rheum. 62, 2047–2054 (2010).
Lertdumrongluk, P. et al. Pharmacokinetics of mycophenolic acid in severe lupus nephritis. Kidney Int. 78, 389–395 (2010).
Luszczynska, P. et al. Pharmacokinetics of free and total mycophenolic acid in adult lupus nephritis patients-implications for therapeutic drug monitoring. Eur. J. Clin. Pharmacol. 75, 371–379 (2019).
Pourafshar, N. et al. The utility of trough mycophenolic acid levels for the management of lupus nephritis. Nephrol. Dial. Transpl. 34, 83–89 (2019).
Mok, C. C. et al. Tacrolimus versus mycophenolate mofetil for induction therapy of lupus nephritis: a randomised controlled trial and long-term follow-up. Ann. Rheum. Dis. 75, 30–36 (2016).
Chen, W. et al. Short-term outcomes of induction therapy with tacrolimus versus cyclophosphamide for active lupus nephritis: a multicenter randomized clinical trial. Am. J. Kidney Dis. 57, 235–244 (2011).
Liao, R. et al. Tacrolimus protects podocytes from injury in lupus nephritis partly by stabilizing the cytoskeleton and inhibiting podocyte apoptosis. PLOS ONE 10, e0132724 (2015).
Zhang, H. et al. Multitarget therapy for maintenance treatment of lupus nephritis. J. Am. Soc. Nephrol. 28, 3671–3678 (2017).
Liu, Z. et al. Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Ann. Intern. Med. 162, 18–26 (2015).
Rovin, B. H. et al. A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int. 95, 219–231 (2019).
Rovin, B. H. et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheum. 64, 1215–1226 (2012). A randomized clinical trial comparing safety and efficacy of rituximab added to the standard-of-care immunosuppressive therapy in the treatment of LN. The study did not meet the primary end points.
Gomez Mendez, L. M. et al. Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis. Clin. J. Am. Soc. Nephrol. 13, 1502–1509 (2018).
Parodis, I. et al. Rituximab-mediated late-onset neutropenia in systemic lupus erythematosus – distinct roles of BAFF and APRIL. Lupus 27, 1470–1478 (2018).
Carter, L. M., Isenberg, D. A. & Ehrenstein, M. R. Elevated serum BAFF levels are associated with rising anti-double-stranded DNA antibody levels and disease flare following B cell depletion therapy in systemic lupus erythematosus. Arthritis Rheum. 65, 2672–2679 (2013).
Navarra, S. V. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 377, 721–731 (2011).
Furie, R. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 63, 3918–3930 (2011).
Dooley, M. et al. Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus 22, 63–72 (2013).
GSK. GSK announces positive headline results in phase 3 study of Benlysta in patients with lupus nephritis. prnewswire.com https://www.prnewswire.com/news-releases/gsk-announces-positive-headline-results-in-phase-3-study-of-benlysta-in-patients-with-lupus-nephritis-300976537.html (2019).
Cao, H. et al. The efficacy and safety of leflunomide for the treatment of lupus nephritis in Chinese patients: systematic review and meta-analysis. PLOS ONE 10, e0144548 (2015).
Grootscholten, C. et al. Azathioprine/methylprednisolone versus cyclophosphamide in proliferative lupus nephritis. A randomized controlled trial. Kidney Int. 70, 732–742 (2006).
Moroni, G., Quaglini, S., Maccario, M., Banfi, G. & Ponticelli, C. “Nephritic flares” are predictors of bad long-term renal outcome in lupus nephritis. Kidney Int. 50, 2047–2053 (1996).
Illei, G. G. et al. Renal flares are common in patients with severe proliferative lupus nephritis treated with pulse immunosuppressive therapy: long-term followup of a cohort of 145 patients participating in randomized controlled studies. Arthritis Rheum. 46, 995–1002 (2002).
Contreras, G. et al. Sequential therapies for proliferative lupus nephritis. N. Engl. J. Med. 350, 971–980 (2004).
Houssiau, F. A. et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann. Rheum. Dis. 69, 61–64 (2010).
Dooley, M. A. et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N. Engl. J. Med. 365, 1886–1895 (2011). A randomized clinical trial showing greater efficacy of MMF than of azathioprine as a maintenance agent in preventing relapses in patients with LN.
Tamirou, F. et al. Long-term follow-up of the MAINTAIN nephritis trial, comparing azathioprine and mycophenolate mofetil as maintenance therapy of lupus nephritis. Ann. Rheum. Dis. 75, 526–531 (2016).
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guidelines for glomerulonephritis. Kidney Int. Suppl. 2, 139–274 (2012).
Palmer, S. C. et al. Induction and maintenance immunosuppression treatment of proliferative lupus nephritis: a network meta-analysis of randomized trials. Am. J. Kidney Dis. 70, 324–336 (2017).
Floege, J. et al. Management and treatment of glomerular diseases (part 1): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 95, 268–280 (2019).
Malvar, A. et al. Kidney biopsy-based management of maintenance immunosuppression is safe and may ameliorate flare rate in lupus nephritis. Kidney Int. 97, 156–162 (2020).
Kraft, S. W., Schwartz, M. M., Korbet, S. M. & Lewis, E. J. Glomerular podocytopathy in patients with systemic lupus erythematosus. J. Am. Soc. Nephrol. 16, 175–179 (2005).
Mok, C., Cheung, T. & Lo, W. Minimal mesangial lupus nephritis: a systematic review. Scand. J. Rheumatol. 39, 181–189 (2010).
Radhakrishnan, J. et al. Mycophenolate mofetil and intravenous cyclophosphamide are similar as induction therapy for class V lupus nephritis. Kidney Int. 77, 152–160 (2010).
Austin, H. A., Illei, G. G., Braun, M. J. & Balow, J. E. Randomized, controlled trial of prednisone, cyclophosphamide, and cyclosporine in lupus membranous nephropathy. J. Am. Soc. Nephrol. 20, 901–911 (2009).
Praga, M., Barrio, V., Juárez, G. F. & Luño, J. Tacrolimus monotherapy in membranous nephropathy: a randomized controlled trial. Kidney Int. 71, 924–930 (2007).
Howman, A. et al. Immunosuppression for progressive membranous nephropathy: a UK randomised controlled trial. Lancet 381, 744–751 (2013).
Fervenza, F. C. et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N. Engl. J. Med. 381, 36–46 (2019).
Jayne, D. et al. Autologous stem cell transplantation for systemic lupus erythematosus. Lupus 13, 168–176 (2004).
Lee, S.-J., Silverman, E. & Bargman, J. M. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat. Rev. Nephrol. 7, 718–729 (2011).
Marmor, M. F., Kellner, U., Lai, T. Y. Y., Lyons, J. S. & Mieler, W. F. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 118, 415–422 (2011).
Kim, J.-W. et al. Risk of retinal toxicity in longterm users of hydroxychloroquine. J. Rheumatol. 44, 1674–1679 (2017).
Melles, R. B. & Marmor, M. F. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 132, 1453–1460 (2014).
Khubchandani, S. R. & Bichle, L. S. Hydroxychloroquine-induced phospholipidosis in a case of SLE: the wolf in zebra clothing. Ultrastruct. Pathol. 37, 146–150 (2013).
Korbet, S. M. et al. Factors predictive of outcome in severe lupus nephritis. Am. J. Kidney Dis. 35, 904–914 (2000).
Chen, Y. E., Korbet, S. M., Katz, R. S., Schwartz, M. M. & Lewis, E. J. Value of a complete or partial remission in severe lupus nephritis. Clin. J. Am. Soc. Nephrol. 3, 46–53 (2008).
Fung, W. A., Su, J. & Touma, Z. Predictors of good long-term renal outcomes in lupus nephritis: results from a single lupus cohort. Biomed. Res. Int. 2017, 5312960 (2017).
Arriens, C. et al. Prognostic significance of repeat biopsy in lupus nephritis: histopathologic worsening and a short time between biopsies is associated with significantly increased risk for end stage renal disease and death. Clin. Immunol. 185, 3–9 (2017).
Narváez, J. et al. The value of repeat biopsy in lupus nephritis flares. Medicine 96, e7099 (2017).
Weng, C.-T. et al. Pneumocystis jirovecii pneumonia in systemic lupus erythematosus from southern Taiwan. J. Clin. Rheumatol. 19, 252–258 (2013).
Park, J. W. et al. Prophylactic effect of trimethoprim-sulfamethoxazole for pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann. Rheum. Dis. 77, 644–649 (2018).
Park, J. W. et al. Pneumocystis pneumonia in patients with rheumatic diseases receiving prolonged, non-high-dose steroids-clinical implication of primary prophylaxis using trimethoprim-sulfamethoxazole. Arthritis Res. Ther. 21, 207 (2019).
Jeffries, M. et al. Sulpha allergy in lupus patients: a clinical perspective. Lupus 17, 202–205 (2008).
Pope, J., Jerome, D., Fenlon, D., Krizova, A. & Ouimet, J. Frequency of adverse drug reactions in patients with systemic lupus erythematosus. J. Rheumatol. 30, 480–484 (2003).
Utsunomiya, M. et al. Optimal regimens of sulfamethoxazole-trimethoprim for chemoprophylaxis of pneumocystis pneumonia in patients with systemic rheumatic diseases: results from a non-blinded, randomized controlled trial. Arthritis Res. Ther. 19, 7 (2017).
Suyama, Y. et al. Safety and efficacy of upfront graded administration of trimethoprim–sulfamethoxazole in systemic lupus erythematosus: a retrospective cohort study. Mod. Rheumatol. 26, 557–561 (2016).
Gaitonde, S. Efficacy of isoniazid prophylaxis in patients with systemic lupus erythematosus receiving long term steroid treatment. Ann. Rheum. Dis. 61, 251–253 (2002).
Liaw, Y.-F. et al. Asian-pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol. Int. 6, 531–561 (2012).
Maillefert, J. F. et al. Rheumatic disorders developed after hepatitis B vaccination. Rheumatology 38, 978–983 (1999).
Maillefert, J. F., Tavernier, C., Sibilia, J. & Vignon, E. Exacerbation of systemic lupus erythematosus after hepatitis B vaccination: comment on the article by Battafarano et al and the letter by Senecal et al. Arthritis Rheum. 43, 468–469 (2000).
Kuruma, K. A., Borba, E. F., Lopes, M. H., de Carvalho, J. F. & Bonfa, E. Safety and efficacy of hepatitis B vaccine in systemic lupus erythematosus. Lupus 16, 350–354 (2007).
Sarin, S. K. et al. Asian-pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol. Int. 10, 1–98 (2016).
Singh, R. R. & Yen, E. Y. SLE mortality remains disproportionately high, despite improvements over the last decade. Lupus 27, 1577–1581 (2018).
Yen, E. Y. et al. 46-Year trends in systemic lupus erythematosus mortality in the United States, 1968 to 2013: a nationwide population-based study. Ann. Intern. Med. 167, 777–785 (2017).
Yurkovich, M., Vostretsova, K., Chen, W. & Avina-Zubieta, J. A. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res. 66, 608–616 (2014).
Rahman, P., Gladman, D. D., Urowitz, M. B., Hallett, D. & Tam, L. S. Early damage as measured by the SLICC/ACR damage index is a predictor of mortality in systemic lupus erythematosus. Lupus 10, 93–96 (2001).
Nikpour, M., Urowitz, M. B. & Gladman, D. D. Premature atherosclerosis in systemic lupus erythematosus. Rheum. Dis. Clin. North Am. 31, 329–354 (2005).
Fasano, S., Pierro, L., Pantano, I., Iudici, M. & Valentini, G. Longterm hydroxychloroquine therapy and low-dose aspirin may have an additive effectiveness in the primary prevention of cardiovascular events in patients with systemic lupus erythematosus. J. Rheumatol. 44, 1032–1038 (2017).
Iudici, M. et al. Low-dose aspirin as primary prophylaxis for cardiovascular events in systemic lupus erythematosus: a long-term retrospective cohort study. Rheumatology 55, 1623–1630 (2016).
Piepoli, M. F. et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 37, 2315–2381 (2016).
Gaziano, J. M. et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 392, 1036–1046 (2018).
Bowman, L. et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N. Engl. J. Med. 379, 1529–1539 (2018).
Petri, M. A., Kiani, A. N., Post, W., Christopher-Stine, L. & Magder, L. S. Lupus atherosclerosis prevention study (LAPS). Ann. Rheum. Dis. 70, 760–765 (2011).
Schanberg, L. E. et al. Use of atorvastatin in systemic lupus erythematosus in children and adolescents. Arthritis Rheum. 64, 285–296 (2012).
Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2, 279–335 (2012).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int. Suppl. 7, 1–59 (2017).
Mok, C. C., Mak, A. & Ma, K. M. Bone mineral density in postmenopausal Chinese patients with systemic lupus erythematosus. Lupus 14, 106–112 (2005).
Uaratanawong, S., Deesomchoke, U., Lertmaharit, S. & Uaratanawong, S. Bone mineral density in premenopausal women with systemic lupus erythematosus. J. Rheumatol. 30, 2365–2368 (2003).
Ramsey-Goldman, R. et al. Frequency of fractures in women with systemic lupus erythematosus: comparison with United States population data. Arthritis Rheum. 42, 882–890 (1999).
Oleksik, A. et al. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J. Bone Miner. Res. 15, 1384–1392 (2000).
Hasserius, R., Karlsson, M. K., Nilsson, B. E., Redlund-Johnell, I. & Johnell, O. Prevalent vertebral deformities predict increased mortality and increased fracture rate in both men and women: a 10-year population-based study of 598 individuals from the Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos. Int. 14, 61–68 (2003).
Buckley, L. et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 69, 1521–1537 (2017).
Buyon, J. P. et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann. Intern. Med. 142, 953–962 (2005).
Mok, C. C., To, C. H., Mak, A. & Ma, K. M. Raloxifene for postmenopausal women with systemic lupus erythematosus: a pilot randomized controlled study. Arthritis Rheum. 52, 3997–4002 (2005).
Crew, R. J., Radhakrishnan, J. & Appel, G. Complications of the nephrotic syndrome and their treatment. Clin. Nephrol. 62, 245–259 (2004).
Lee, T. et al. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int. 85, 1412–1420 (2014).
Mercadal, L. et al. Factors affecting outcome and prognosis in membranous lupus nephropathy. Nephrol. Dial. Transpl. 17, 1771–1778 (2002).
Mantha, S. et al. Assessing the risk of venous thromboembolic events in women taking progestin-only contraception: a meta-analysis. Br. Med. J. 345, e4944 (2012).
Mok, C. C., Lau, C. S. & Wong, R. W. Risk factors for ovarian failure in patients with systemic lupus erythematosus receiving cyclophosphamide therapy. Arthritis Rheum. 41, 831–837 (1998).
Somers, E. C., Marder, W., Christman, G. M., Ognenovski, V. & McCune, W. J. Use of a gonadotropin-releasing hormone analog for protection against premature ovarian failure during cyclophosphamide therapy in women with severe lupus. Arthritis Rheum. 52, 2761–2767 (2005).
Tamirou, F. et al. Brief report: the Euro-Lupus low-dose intravenous cyclophosphamide regimen does not impact the ovarian reserve, as measured by serum levels of anti-Müllerian hormone. Arthritis Rheumatol. 69, 1267–1271 (2017).
Wasserman, S. & Clowse, M. E. B. in Contraception and Pregnancy in Patients with Rheumatic Disease (eds Sammaritano, L. R. & Bermas, B. L.) 79–97 (Springer, 2014).
Clowse, M. E. B., Magder, L. S., Witter, F. & Petri, M. Early risk factors for pregnancy loss in lupus. Obstet. Gynecol. 107, 293–299 (2006).
Liu, J. et al. Pregnancy in women with systemic lupus erythematosus: a retrospective study of 111 pregnancies in Chinese women. J. Materrn. Fetal Neonatal Med. 25, 261–266 (2012).
Clowse, M. E. B., Magder, L. S., Witter, F. & Petri, M. The impact of increased lupus activity on obstetric outcomes. Arthritis Rheum. 52, 514–521 (2005).
Smyth, A. et al. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin. J. Am. Soc. Nephrol. 5, 2060–2068 (2010).
Ruffatti, A. et al. Antibody profile and clinical course in primary antiphospholipid syndrome with pregnancy morbidity. Thromb. Haemost. 96, 337–341 (2006).
Buyon, J. P. et al. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann. Intern. Med. 163, 153–163 (2015). This large prospective multiethnic study provides an approach to identify pregnant patients with SLE who are at risk of adverse outcomes.
Clowse, M. E. B. & Grotegut, C. Racial and ethnic disparities in the pregnancies of women with systemic lupus erythematosus: lupus pregnancy and racial disparities. Arthritis Care Res. 68, 1567–1572 (2016).
Skorpen, C. G. et al. Influence of disease activity and medications on offspring birth weight, pre-eclampsia and preterm birth in systemic lupus erythematosus: a population-based study. Ann. Rheum. Dis. 77, 264–269 (2018). This study compares the frequency of serious adverse outcomes in pregnancy in patients with active and inactive SLE with the general population in a national registry and emphasizes that active SLE enhances the risk of these outcomes.
Izmirly, P. M., Rivera, T. L. & Buyon, J. P. Neonatal lupus syndromes. Rheum. Dis. Clin. North Am. 33, 267–285 (2007).
Saavedra, M. A. et al. Impact of previous lupus nephritis on maternal and fetal outcomes during pregnancy. Clin. Rheumatol. 31, 813–819 (2012).
Wagner, S. et al. Maternal and foetal outcomes in pregnant patients with active lupus nephritis. Lupus 18, 342–347 (2009).
Bramham, K. et al. Pregnancy outcomes in systemic lupus erythematosus with and without previous nephritis. J. Rheumatol. 38, 1906–1913 (2011).
Palmsten, K. et al. Patterns of prednisone use during pregnancy in women with rheumatoid arthritis: daily and cumulative dose. Pharmacoepidemiol. Drug Saf. 27, 430–438 (2018).
Meads, C. et al. Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol. Assess. 12, iii–270 (2008).
ACOG Committee Opinion No. 743: low-dose aspirin use during pregnancy. Obstet. Gynecol. 132, e44–e52 (2018).
Ware, J. E. & Sherbourne, C. D. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med. Care 30, 473–483 (1992).
Wang, C., Mayo, N. E. & Fortin, P. R. The relationship between health related quality of life and disease activity and damage in systemic lupus erythematosus. J. Rheumatol. 28, 525–532 (2001).
EuroQol Group. EuroQol — a new facility for the measurement of health-related quality of life. Health Policy 16, 199–208 (1990).
Wolfe, F., Michaud, K., Li, T. & Katz, R. S. EQ-5D and SF-36 quality of life measures in systemic lupus erythematosus: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, and fibromyalgia. J. Rheumatol. 37, 296–304 (2010).
Webster, K., Cella, D. & Yost, K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual. Life Outcomes 1, 79 (2003).
Kosinski, M., Gajria, K., Fernandes, A. & Cella, D. Qualitative validation of the FACIT-fatigue scale in systemic lupus erythematosus. Lupus 22, 422–430 (2013).
Leong, K. P. et al. Development and preliminary validation of a systemic lupus erythematosus-specific quality-of-life instrument (SLEQOL). Rheumatology 44, 1267–1276 (2005).
McElhone, K. et al. Development and validation of a disease-specific health-related quality of life measure, the LupusQol, for adults with systemic lupus erythematosus. Arthritis Rheum. 57, 972–979 (2007).
Jolly, M. et al. Disease-specific patient reported outcome tools for systemic lupus erythematosus. Semin. Arthritis Rheum. 42, 56–65 (2012).
Jolly, M. How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J. Rheumatol. 32, 1706–1708 (2005).
Mok, C. C. Treat-to-target in systemic lupus erythematosus: are we there yet? Expert. Rev. Clin. Pharmacol. 9, 675–680 (2016).
van Vollenhoven, R. F. et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann. Rheum. Dis. 73, 958–967 (2014).
Mazzoni, D., Cicognani, E. & Prati, G. Health-related quality of life in systemic lupus erythematosus: a longitudinal study on the impact of problematic support and self-efficacy. Lupus 26, 125–131 (2017). This study indicates that the HRQOL of patients with SLE is influenced by self-efficacy in the management of the disease and problematic support.
Williams, E. M. et al. Intervention to improve quality of life for African-American lupus patients (IQAN): study protocol for a randomized controlled trial of a unique a la carte intervention approach to self-management of lupus in African Americans. BMC Health Serv. Res. 16, 339 (2016).
Yazdany, J. & Yelin, E. Health-related quality of life and employment among persons with systemic lupus erythematosus. Rheum. Dis. Clin. North Am. 36, 15–32 (2010).
Kuriya, B., Gladman, D. D., Ibañez, D. & Urowitz, M. B. Quality of life over time in patients with systemic lupus erythematosus. Arthritis Rheum. 59, 181–185 (2008).
Friedman, A. W. et al. Systemic lupus erythematosus in three ethnic groups. IV. Factors associated with self-reported functional outcome in a large cohort study. Arthritis Care Res. 12, 256–266 (1999).
Thumboo, J. et al. A prospective study of factors affecting quality of life in systemic lupus erythematosus. J. Rheumatol. 27, 1414–1420 (2000).
Devins, G. M. & Edworthy, S. M. Illness intrusiveness explains race-related quality-of-life differences among women with systemic lupus erythematosus. Lupus 9, 534–541 (2000).
Doria, A. Health-related quality of life in Italian patients with systemic lupus erythematosus. II. Role of clinical, immunological and psychological determinants. Rheumatology 43, 1580–1586 (2004).
Dobkin, P. L. et al. Quality of life in systemic lupus erythematosus patients during more and less active disease states: differential contributors to mental and physical health. Arthritis Care Res. 12, 401–410 (1999).
Medeiros, M. M. C. et al. Health-related quality of life in patients with systemic lupus erythematosus and its relationship with cyclophosphamide pulse therapy. Eur. J. Intern. Med. 19, 122–128 (2008).
Jolly, M. et al. Disease-specific quality of life in patients with lupus nephritis. Lupus 27, 257–264 (2018). These international, cross-sectional data from a large cohort of patients with SLE demonstrate that patients with active LN have worse quality of life than those with inactive LN.
Chaigne, B. et al. Impact of disease activity on health-related quality of life in systemic lupus erythematosus — a cross-sectional analysis of the Swiss Systemic Lupus Erythematosus Cohort Study (SSCS). BMC Immunol. 18, 17 (2017). This cross-sectional study of the Swiss SLE Cohort indicates that the impact of disease activity on HRQOL dimensions depends on specific Safety of Estrogens in Lupus National Assessment (SELENA)-Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) system components, namely, musculoskeletal, renal and immunological manifestations of the disease.
Vu, T. V. & Escalante, A. A comparison of the quality of life of patients with systemic lupus erythematosus with and without endstage renal disease. J. Rheumatol. 26, 2595–2601 (1999).
Clarke, A. E. et al. SLE patients with renal damage incur higher health care costs. Rheumatology 47, 329–333 (2007).
Appenzeller, S. et al. The relationship between renal activity and quality of life in systemic lupus erythematosus. J. Rheumatol. 36, 947–952 (2009).
Daleboudt, G. M. N., Berger, S. P., Broadbent, E. & Kaptein, A. A. Health-related quality of life in patients with systemic lupus erythematosus and proliferative lupus nephritis. Psychol. Health Med. 16, 393–404 (2011).
Tse, K. C., Tang, C. S., Lio, W. I., Lam, M. F. & Chan, T. M. Quality of life comparison between corticosteroid-and-mycofenolate mofetil and corticosteroid-and-oral cyclophosphamide in the treatment of severe lupus nephritis. Lupus 15, 371–379 (2006).
Grootscholten, C. et al. Health-related quality of life and treatment burden in patients with proliferative lupus nephritis treated with cyclophosphamide or azathioprine/methylprednisolone in a randomized controlled trial. J. Rheumatol. 34, 1699–1707 (2007).
Parodis, I. et al. The impact of belimumab and rituximab on health-related quality of life in patients with systemic lupus erythematosus. Arthritis Care Res. 71, 811–821 (2019).
Alarcón-Riquelme, M. E. New attempts to define and clarify lupus. Curr. Rheumatol. Rep. 21, 11 (2019).
Mysler, E. F. et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study: ocrelizumab in lupus nephritis. Arthritis Rheum. 65, 2368–2379 (2013).
Furie, R. et al. Efficacy and safety of abatacept in lupus nephritis: a twelve-month, randomized, double-blind study: abatacept in lupus nephritis. Arthritis Rheumatol. 66, 379–389 (2014).
Isenberg, D. et al. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann. Rheum. Dis. 74, 2006–2015 (2015).
Furie, R. et al. in Annual European Congress of Rheumatology, EULAR 2018, Amsterdam, 13–16 June 176–177 (BMJ and European League Against Rheumatism, 2018).
Rovin, B. et al. A phase 2 randomized, controlled study of obinutuzumab with mycophenolate and corticosteroids inproliferative lupus nephritis (abstract FR-OR136). Presented at Proc. American Society of Nephrology Kidney Week 2019.
Alunno, A., Padjen, I., Fanouriakis, A. & Boumpas, D. T. Pathogenic and therapeutic relevance of JAK/STAT signaling in systemic lupus erythematosus: integration of distinct inflammatory pathways and the prospect of their inhibition with an oral agent. Cells 8, E898 (2019).
Anders, H.-J., Jayne, D. R. W. & Rovin, B. H. Hurdles to the introduction of new therapies for immune-mediated kidney diseases. Nat. Rev. Nephrol. 12, 205–216 (2016).
Jayne, D. R. W. et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J. Am. Soc. Nephrol. 28, 2756–2767 (2017).
Houssiau, F. A. Time to change the primary outcome of lupus trials. Ann. Rheum. Dis. 78, 581–582 (2019).
van Vollenhoven, R. et al. A framework for remission in SLE: consensus findings from a large international task force on definitions of remission in SLE (DORIS). Ann. Rheum. Dis. 76, 554–561 (2017).
Anders, H.-J. & Rovin, B. A pathophysiology-based approach to the diagnosis and treatment of lupus nephritis. Kidney Int. 90, 493–501 (2016).
Acknowledgements
H.-J.A. was supported by the Deutsche Forschungsgemeinschaft (AN372/24-1). I.P. was supported by the Swedish Rheumatism Association (R-859621), the Professor Nanna Svartz Foundation (2018-00250), the Ulla and Roland Gustafsson Foundation (2019-12), Region Stockholm and the Karolinska Institutet. M.-h.Z. was supported by funding from the National Natural Science Foundation of China to the Innovation Research Group (81621092). R.S. was supported by the George M. O’Brien Kidney Research Core Center (US National Institutes of Health grant P30DK079328). C.M. was supported by the US National Institutes of Health grant RO1 AR074096. The authors thank T. Nguyen and A.S.C.L.S. Titus for editorial support.
Author information
Authors and Affiliations
Contributions
Introduction (C.M., H.-J.A., R.S., I.P. and J.E.S.); Epidemiology (M.-h.Z.); Mechanisms/pathophysiology (C.M. and H.-J.A.); Diagnosis, screening and prevention (I.P. and M.-h.Z.); Management (R.S. and J.E.S.); Quality of life (M.-h.Z.); Outlook (C.M., H.-J.A., R.S., M.-h.Z., I.P. and J.E.S.); Overview of Primer (C.M., H.-J.A. and I.P.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
co-senior authors: Hans-Joachim Anders, Ramesh Saxena, Ming-hui Zhao, Ioannis Parodis, Jane E. Salmon and Chandra Mohan
Rights and permissions
About this article
Cite this article
Anders, HJ., Saxena, R., Zhao, Mh. et al. Lupus nephritis. Nat Rev Dis Primers 6, 7 (2020). https://doi.org/10.1038/s41572-019-0141-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-019-0141-9