Abstract
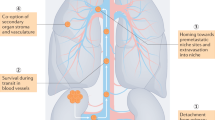
Approximately 70% of cases of kidney cancer are localized or locally advanced at diagnosis. Among patients who undergo surgery for these cancers, 30–35% will eventually develop potentially fatal metachronous distant metastases. Effective adjuvant treatments are urgently needed to reduce the risk of recurrence of kidney cancer and of dying of metastatic disease. To date, almost all of the tested adjuvant agents have failed to demonstrate any benefit. Only two trials of an autologous renal tumour cell vaccine and of the vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor sunitinib have shown positive results, but these have been criticized for methodological reasons and conflicting data, respectively. The results of two additional trials of targeted agents as adjuvant therapies have not yet been published. Novel immune checkpoint inhibitors are promising approaches to adjuvant therapy in kidney cancer, and a number of trials are now underway. An important component of the management of patients with kidney cancer, particularly those who undergo radical resection for localized renal cell carcinoma, is the preservation of kidney function to reduce morbidity and mortality. The optimal management of these patients therefore requires a multidisciplinary approach involving nephrologists, oncologists, urologists and pathologists.
Key points
-
Effective adjuvant treatments for kidney cancer are needed to reduce the risk of recurrence and of dying of metastatic disease.
-
To date, almost all of the tested adjuvant agents have failed to demonstrate any benefit in clinical trials; the two positive trials were criticized for methodological reasons and conflicting results.
-
Only one drug — sunitinib — has been approved for the adjuvant treatment of kidney cancer in the USA; however, this drug has not been approved as an adjuvant therapy in Europe.
-
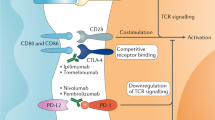
Positive results with immune checkpoint inhibitors in metastatic renal cell carcinoma (RCC) suggest that these agents might also be effective adjuvant therapies; trials of these agents are underway.
-
Preservation of kidney function in patients with RCC is important to reduce morbidity; therefore, multidisciplinary management should be mandatory for almost all patients with radically resected kidney cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 (2015).
American Cancer Society. Key statistics about kidney cancer. cancer.org https://www.cancer.org/cancer/kidney-cancer/about/key-statistics.html (updated 4 Jan 2018).
Lam, J. S., Leppert, J. T., Figlin, R. A. & Belldegrun, A. S. Surveillance following radical or partial nephrectomy for renal cell carcinoma. Curr. Urol. Rep. 6, 7–18 (2005).
Gupta, K., Miller, J. D., Li, J. Z., Russel, M. W. & Charbonneau, C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat. Rev. 34, 193–205 (2008).
American Cancer Society. Survival rates for kidney cancer. cancer.org https://www.cancer.org/cancer/kidney-cancer/detection-diagnosis-staging/survival-rates.html (updated 31 Jan 2019).
Massari, F. et al. Adjuvant therapy in renal cell carcinoma. Cancer Treat. Rev. 60, 152–157 (2017).
Porta, C., Chiellino, S., Ferrari, A., Mariucci, S. & Liguigli, W. Pharmacotherapy for treating metastatic clear cell renal cell carcinoma. Expert Opin. Pharmacother. 18, 205–216 (2017).
Zisman, A. et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J. Clin. Oncol. 19, 1649–1657 (2001).
Leibovich, B. C. et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 97, 1663–1671 (2003).
Tan, M. H. et al. Comparison of the UCLA Integrated Staging System and the Leibovich score in survival prediction for patients with nonmetastatic clear cell renal cell carcinoma. Urology 75, 1365–1370 (2010).
Frank, I. et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J. Urol. 168, 2395–2400 (2002).
Karakiewicz, P. I. et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J. Clin. Oncol. 25, 1316–1322 (2007).
Kattan, M. W., Reuter, V., Motzer, R. J., Katz, J. & Russo, P. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 166, 63–67 (2001).
Pal, S. K. & Haas, N. B. Adjuvant therapy for renal cell carcinoma: past, present, and future. Oncologist 19, 851–859 (2014).
Brooks, S. A. et al. ClearCode34: a prognostic risk predictor for localized clear cell renal cell carcinoma. Eur. Urol. 66, 77–84 (2014).
Brannon, A. R. et al. Molecular stratification of clear cell renal cell carcinoma by consensus clustering reveals distinct subtypes and survival patterns. Genes Cancer 1, 152–163 (2010).
Rini, B. et al. A 16-gene assay to predict recurrence after surgery in localised renal cell carcinoma: development and validation studies. Lancet Oncol. 16, 676–685 (2015).
Kapur, P. et al. Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: a retrospective analysis with independent validation. Lancet Oncol. 14, 159–167 (2013).
Kjaer, M. et al. A randomized trial of postoperative radiotherapy versus observation in stage II and III renal adenocarcinoma. A study by the Copenhagen Renal Cancer Study Group. Scand. J. Urol. Nephrol. 21, 285–289 (1987).
Pizzocaro, G. et al. Interferon adjuvant to radical nephrectomy in Robson stages II and III renal cell carcinoma: a multicentric randomized study. J. Clin. Oncol. 19, 425–431 (2001).
Messing, E. M. et al. Phase III study of interferon alfa-NL as adjuvant treatment for resectable renal cell carcinoma: an Eastern Cooperative Oncology Group/Intergroup trial. J. Clin. Oncol. 21, 1214–1222 (2003).
Clark, J. I. et al. Adjuvant high-dose bolus interleukin-2 for patients with high-risk renal cell carcinoma: a Cytokine Working Group randomized trial. J. Clin. Oncol. 21, 3133–3140 (2003).
Atzpodien, J. et al. Adjuvant treatment with interleukin-2- and interferon-alpha2a-based chemoimmunotherapy in renal cell carcinoma post tumour nephrectomy: results of a prospectively randomised trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). Br. J. Cancer 92, 843–846 (2005).
Passalacqua, R. et al. Adjuvant low-dose Interleukin-2 (IL-2) plus Interferon-α (IFN-α) in operable renal cell carcinoma (RCC): a phase III, randomized, multicentre trial of the Italian Oncology Group for Clinical Research (GOIRC). J. Immunother. 37, 440–447 (2014).
Aitchison, M. et al. Adjuvant 5-flurouracil, alpha-interferon and interleukin-2 versus observation in patients at high risk of recurrence after nephrectomy for renal cell carcinoma: results of a phase III randomised European Organisation for Research and Treatment of Cancer (Genito-Urinary Cancers Group)/National Cancer Research Institute trial. Eur. J. Cancer 50, 70–77 (2014).
Adler, A. et al. Active specific immunotherapy of renal cell carcinoma patients: a prospective randomized study of hormono-immuno-versus hormonotherapy. Preliminary report of immunological and clinical aspects. J. Biol. Response Mod. 6, 610–624 (1987).
Galligioni, E. et al. Adjuvant immunotherapy treatment of renal carcinoma patients with autologous tumor cells and bacillus Calmette-Guèrin: five-year results of a prospective randomized study. Cancer 77, 2560–2566 (1996).
Jocham, D. et al. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet 363, 594–599 (2004). This trial is the only formally positive study of adjuvant therapy in RCC; however, it has been heavily criticized.
Wood, C. et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet 372, 145–154 (2008).
Pizzocaro, G. et al. Adjuvant medroxyprogesterone acetate to radical nephrectomy in renal cancer: 5-year results of a prospective randomized study. J. Urol. 138, 1379–1381 (1987).
Naito, S. et al. Postoperative UFT adjuvant and the risk factors for recurrence in renal cell carcinoma: a long-term follow-up study. Kyushu University Urological Oncology Group. Int. J. Urol. 4, 8–12 (1997).
Margulis, V. et al. Randomized trial of adjuvant thalidomide versus observation in patients with completely resected high-risk renal cell carcinoma. Urology 73, 337–341 (2009).
Chamie, K. et al. Adjuvant weekly girentuximab following nephrectomy for high-risk renal cell carcinoma: the ARISER randomized clinical trial. JAMA Oncol. 3, 913–920 (2017).
Supuran, C. T. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 7, 48 (2017).
Kramar, A. et al. Guidelines for the definition of time-to-event end points in renal cell cancer clinical trials: results of the DATECAN project. Ann. Oncol. 26, 2392–2398 (2015).
Massari, F. et al. Adjuvant treatment for resected renal cell carcinoma: are all strategies equally negative? Potential implications for trial design with targeted agents. Clin. Genitourin. Cancer 11, 471–476 (2013).
Gnarra, J. R. et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat. Genet. 7, 85–90 (1994).
Shuin, T. et al. Frequent somatic mutations and loss of heterozygosity of the von Hippel-Lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res. 54, 2852–2855 (1994).
Herman, J. G. et al. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc. Natl Acad. Sci. USA 91, 9700–9704 (1994).
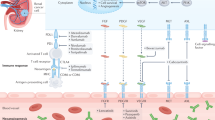
Gruber, M. & Simon, M. C. Hypoxia-inducible factors, hypoxia, and tumor angiogenesis. Curr. Opin. Hematol. 13, 169–174 (2006).
Shen, C. & Kaelin, W. G. The VHL/HIF axis in clear cell renal carcinoma. Semin. Cancer Biol. 23, 18–25 (2013).
Haas, N. B. et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 387, 2008–2016 (2016). This trial of a targeted agent as adjuvant therapy reports negative results.
Ravaud, A. et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N. Engl. J. Med. 375, 2246–2254 (2016). This adjuvant study of sunitinib in RCC is positive in terms of DFS (its primary end point) but not in terms of OS.
Motzer, R. J. et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J. Clin. Oncol. 35, 3916–3923 (2017). This trial of a targeted agent as adjuvant therapy also reports negative results.
Gross-Goupil, M. et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III randomized ATLAS trial. Ann. Oncol. 29, 2371–2378 (2018).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT00492258 (2013). This is the latest negative trial investigating a VEGFR TKI.
U.S. Food and Drug Administration. FDA approves sunitinib malate for adjuvant treatment of renal cell carcinoma. FDA.gov https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm585686.htm (updated 16 Aug 2018).
Haas, N. B. et al. Adjuvant treatment for high-risk clear cell renal cancer: updated results of a high-risk subset of the ASSURE randomized trial. JAMA Oncol. 3, 1249–1252 (2017).
Sternberg, C. et al. Pazopanib exposure relationship with clinical efficacy and safety in the adjuvant treatment of advanced renal cell carcinoma. Clin. Cancer Res. 24, 3005–3013 (2018).
Bex, A. et al. Updated European Association of Urology guidelines regarding adjuvant therapy for renal cell carcinoma. Eur. Urol. 71, 719–722 (2017).
European Medicines Agency. Withdrawal assessment report: Sutent. ema.europa.eu https://www.ema.europa.eu/documents/withdrawal-report/withdrawal-assessment-report-sutent_en.pdf (2018).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01120249 (2018).
Vasudev, N. S. & Reynolds, A. R. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis 17, 471–494 (2014).
Kim, B. J. et al. The role of targeted agents in the adjuvant treatment of colon cancer: a meta-analysis of randomized phase III studies and review. Oncotarget 8, 31112–31118 (2017).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378, 1277–1290 (2018). This trial establishes a new standard of care for the first-line treatment of metastatic RCC.
Motzer, R. J. et al. IMmotion151: a randomized phase III study of atezolizumab plus bevacizumab versus sunitinib in untreated metastatic Renal Cell Carcinoma (mRCC) [abstract]. J. Clin. Oncol. 36 (Suppl. 6), 578 (2018).
Motzer, R. J. et al. JAVELIN Renal 101: a randomized, phase 3 study of avelumab+axitinib versus sunitinib as first-line treatment of advanced renal cell carcinoma (aRCC) [abstract]. Ann. Oncol. 29 (Suppl. 8), LBAA6_PR (2018).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03055013 (2019).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03024996 (2019).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03142334 (2019).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03288532 (2018).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03138512 (2019).
Liu, J. et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 6, 1382–1399 (2016).
Hung, P. H. et al. Increased risk of end-stage renal disease in patients with renal cell carcinoma: a 12-year nationwide follow-up study. Medicine (Baltimore) 93, e52 (2014).
Barlow, L. J., Korets, R., Laudano, M., Benson, M. & McKiernan, J. Predicting renal functional outcomes after surgery for renal cortical tumours: a multifactorial analysis. BJU Int. 106, 489–492 (2010).
Jeon, H. G., Jeong, I. G., Lee, J. W., Lee, S. E. & Lee, E. Prognostic factors for chronic kidney disease after curative surgery in patients with small renal tumors. Urology 74, 1064–1068 (2009).
Li, L. et al. Risk of chronic kidney disease after cancer nephrectomy. Nat. Rev. Nephrol. 10, 135–145 (2014).
Cho, A. et al. Post-operative acute kidney injury in patients with renal cell carcinoma is a potent risk factor for new-onset chronic kidney disease after radical nephrectomy. Nephrol. Dial. Transplant. 26, 3496–3501 (2011).
Lam, A. Q. & Humphreys, B. D. Onco-nephrology: AKI in the cancer patient. Clin. J. Am. Soc. Nephrol. 7, 1692–1700 (2012).
Gallieni, M. et al. Acute kidney injury in cancer patients. Contrib. Nephrol. 13, 137–148 (2018).
Cosmai, L. et al. Opening an onconephrology clinic: recommendations and basic requirements. Nephrol. Dial. Transplant. 33, 1503–1510 (2018). This paper discusses the requirements needed to run an onco-nephrology clinic as well as its field of interest.
Taylor, A. T. Radionuclides in nephrourology, part 2: pitfalls and diagnostic applications. Nucl. J. Med. 55, 786–798 (2014).
Srigley, J. R. et al. Protocol for the examination of specimens from patients with invasive carcinoma of renal tubular origin. Arch. Pathol. Lab. Med. 134, e25–e30 (2010).
Algaba, F. et al. Handling and reporting of nephrectomy specimens for adult renal tumors: a survey by the European Network of Uropathology. J. Clin. Pathol. 65, 106–113 (2012).
Gupta, S. et al. Safety and efficacy of molecularly targeted agents in patients with metastatic kidney cancer with renal dysfunction. Anticancer Drugs. 22, 794–800 (2011).
Nouhaud, F. X. et al. Baseline chronic kidney disease is associated with toxicity and survival in patients treated with targeted therapies for metastatic renal cell carcinoma. Anticancer Drugs. 26, 866–871 (2015).
Zabor, E. C. et al. Factors associated with recovery of renal function following radical nephrectomy for kidney neoplasm. Clin. J. Am. Soc. Nephrol. 11, 101–107 (2016).
Huang, W. C. et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 7, 735–740 (2006).
Calvo, E. et al. Improvement in survival end points of patients with metastatic renal cell carcinoma through sequential targeted therapy. Cancer Treat. Rev. 50, 109–117 (2016).
Escudier, B. et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27 (Suppl. 5), v58–v68 (2016).
Acknowledgements
Reviewer information
Nature Reviews Nephrology thanks H. Hammers, M. H. Rosner and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors researched the data, contributed to discussions of the content, wrote the article and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.P. and A.B. contributed to the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) discussion regarding approval of sunitinib as an adjuvant treatment for resected renal cell carcinoma. The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Porta, C., Cosmai, L., Leibovich, B.C. et al. The adjuvant treatment of kidney cancer: a multidisciplinary outlook. Nat Rev Nephrol 15, 423–433 (2019). https://doi.org/10.1038/s41581-019-0131-x
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-019-0131-x
This article is cited by
-
The integrate profiling of single-cell and spatial transcriptome RNA-seq reveals tumor heterogeneity, therapeutic targets, and prognostic subtypes in ccRCC
Cancer Gene Therapy (2024)
-
Extracellular vesicle-circEHD2 promotes the progression of renal cell carcinoma by activating cancer-associated fibroblasts
Molecular Cancer (2023)
-
Regulator of G protein signaling-1 regulates immune infiltration and macrophage polarization in clear cell renal cell carcinoma
International Urology and Nephrology (2023)
-
PDCD5 inhibits progression of renal cell carcinoma by promoting T cell immunity: with the involvement of the HDAC3/microRNA-195-5p/SGK1
Clinical Epigenetics (2022)
-
The roles of metabolic profiles and intracellular signaling pathways of tumor microenvironment cells in angiogenesis of solid tumors
Cell Communication and Signaling (2022)