Abstract
The precise clinicopathologic significance of myeloid differentiation primary response gene (MYD88) L265P mutation in diffuse large B-cell lymphomas (DLBCLs) remains elusive. To investigate the frequency and clinicopathologic significance of the MYD88 L265P mutation in DLBCLs, we conducted a meta-analysis of 40 published studies on 2736 DLBCL patients. We collected relevant published research findings identified using the PubMed and Embase databases. The effect sizes of outcome parameters were calculated using a random-effects model. In this meta-analysis, the MYD88 L265P mutation in DLBCL showed a significant difference according to tumor sites. The overall incidence of the MYD88 L265P mutation in DLBCLs, excluding the central nervous system and testicular DLBCLs, was 16.5%. Notably, the MYD88 L265P mutation rates of CNS and testicular DLBCL patients were 60% and 77%, respectively. Interestingly, the MYD88 L265P mutation was more frequently detected in activated B-cell-like (ABC) or non-germinal center B-cell-like (GCB) than GCB subtype (OR = 3.414, p < 0.001). The MYD88 L265P mutation was significantly associated with old age and poor overall survival, but not with sex and clinical stage. This pooled analysis demonstrates that the MYD88 L265P mutation is significantly associated with the tumor sites and molecular subtypes in DLBCL patients.
Similar content being viewed by others
Introduction
Myeloid differentiation primary response gene (MYD88) is an adaptor protein that activates the nuclear transcription factor κB (NF-κB) signaling through most of the Toll-like receptors (TLRs)1. An L265P mutation, a change from leucine (CTC) to proline (CCG), in the MYD88 Toll/interleukin (IL)-1 receptor domain, recruits MYD88 protien to the cytoplasmic tail of TLRs to form an active complex. The complex promotes NF-κB and Janus kinase-signal transducer and activator of transcription 3 (JAK-STAT3) signaling1.
Recently, many investigators have reported that the prevalence of MYD88 L265P mutation ranges from 0% to 94% in different series of diffuse large B-cell lymphoma (DLBCL) patients2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41. However, since the MYD88 L265P mutation occurs at various frequencies of DLBCL, a general consensus on clinicopathologic implications has not been reached. DLBCL is a heterogeneous non-Hodgkin’s lymphoma mainly comprising molecular subtypes such as germinal center B-cell-like (GCB) and activated B-cell-like (ABC) types42. Several studies have suggested that the frequency of MYD88 L265P mutation may vary depending on the tumor site or molecular subtype of DLBCL2, 8, 21, 24, 30, 41, but individual studies with different designs hinder clear conclusions. Furthermore, the clinicopathologic significance of the MYD88 L265P mutation in each DLBCL patient was controversial.
To address these controversies, we conducted a meta-analysis to examine the frequency of MYD88 L265P mutation and the relationship between this mutation and the clinicopathologic parameters of DLBCL patients.
Results
Prevalence of MYD88 L265P mutation in diffuse large B-cell lymphoma
On pooled analysis of 40 studies, including 2736 DLBCL patients, the overall prevalence rate of MYD88 L265P mutation was 29.0% [95% confidence interval (CI): 17.2–44.5%] (Table 1)2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41. Twenty-nine studies have reported that the frequency of MYD88 L265P mutation in 2285 DLBCL patients except for central nervous system (CNS) and testicular lymphomas was 16.5% (95% CI: 11.9–22.6%)2, 3, 6, 7, 9,10,11, 13, 16,17,18,19,20,21,22, 24, 25, 27, 28, 30, 31, 33,34,35,36,37,38,39, 41. Thirteen4, 5, 8, 9, 12, 14, 15, 18, 21, 23, 26, 32, 40 and four8, 9, 21, 29 studies reported the prevalence of MYD88 L265P mutation in 378 CNS and 88 testicular DLBCL patients. The MYD88 L265P mutation in the CNS and testis were detected in 59.8% (95% CI: 42.2–75.2%) and 77.1% (95% CI: 67.1–84.7%), respectively. The prevalence of MYD88 L265P mutation in CNS and testicular DLBCL was significantly higher than that of DLBCL in other sites (p < 0.001, Q = 49.671, I 2 = 95.974). As a result of subgroup analysis, the prevalence of MYD88 L265P mutation in DLBCL patients was not significantly different according to the race (Supplementary Table S1).
Relationship between MYD88 L265P mutation and clinical parameters of DLBCL
In this study, we analyzed the relationship between MYD88 L265P mutation and the clinical features of DLBCL patients except for CNS DLBCL and primary cutaneous DLBCL, leg type, because these two subtypes are clinically different from other DLBCL patients.
Seven studies have described the association of MYD88 L265P mutation with age9, 11, 17, 18, 33, 34, 37. The MYD88 L265P mutation was detected in 96 (21%) of 452 patients of more than 60 years and in 43 (13%) of 320 patients of 60 years or less. The MYD88 L265P mutation was significantly related to older age (odds ratio (OR) = 1.768; 95% CI: 1.168–2.677; p = 0.007, Q = 3.918, I 2 = 0.000) (Fig. 1).
Six studies presented the association between MYD88 L265P mutation and patient’s sex9, 11, 17, 18, 33, 34. The MYD88 L265P mutation was found in 69 (17%) of 411 male and in 35 (11%) of 315 female patients. No association was found between the MYD88 L265P mutation and sex (OR = 1.566; 95% CI: 0.996–2.464; p = 0.052, Q = 4.288, I 2 = 0.000).
Eighteen studies reported that MYD88 L265P mutation was detected in 255 (21%) of 1236 activated B-cell-like (ABC) or non-germinal center B-cell-like (non-GCB) subtype and in 44 (6%) of 766 GCB subtype patients2, 6, 7, 9, 11, 16, 18, 21, 24, 25, 27, 30, 34, 36,37,38,39, 41. The MYD88 L265P mutation was significantly associated with ABC or non-GCB subtype (OR = 3.414; 95% CI: 2.243–5.195; p < 0.001, Q = 19.986, I 2 = 14.865) (Fig. 2).
Six studies addressed the relationship between MYD88 L265P mutation and clinical stage (III, IV versus I, II)9, 11, 17, 18, 34, 37. The MYD88 L265P mutation was detected in 59 (18%) of 336 high-stage and in 101 (22%) of 465 low-stage cases. There was no association between the MYD88 L265P mutation and clinical stage (OR = 1.244; 95% CI: 0.804–1.924; p = 0.327, Q = 2.377, I 2 = 0.000).
Four studies described the association between MYD88 L265P mutation and international prognostic index (IPI) risk group9, 11, 18, 34. The MYD88 L265P mutation was found in 39 (17%) of 233 cases with the high-risk group and in 54 (13%) of 427 cases with the low-risk group. No relationship was seen between the MYD88 L265P mutation and IPI risk group (OR = 1.522; 95% CI: 0.939–2446; p = 0.088; Q = 2.883, I 2 = 0.000). However, sensitivity analysis revealed that Kim et al.18 study affected the pooled OR. Therefore, in the case of excluding the study by Kim et al.18, the MYD88 L265P mutation was significantly related to high IPI risk group (OR = 1.828; 95% CI: 1.061–3.147; p = 0.030).
Survival analysis
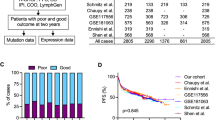
Four studies showed accurate multivariate hazard ratios (HRs) and CIs in 436 DLBCL patients including CNS DLBCL cases (Table 2)11, 15, 18, 31. In this meta-analysis, the estimated adjusted HRs ranged from 0.57 to 3.448. No association was found between the MYD88 L265P mutation and overall survival outcomes (HR = 2.029; 95% CI: 0.873–4.713; p = 0.100, Q = 10.389, I 2 = 71.125).
However, we reassessed the relationship between MYD88 L265P mutation and overall survival of DLBCL in detail based on the subgroup and sensitivity analyses. As a first step, the subgroup analysis revealed that the tumor site of origin (CNS vs. non-CNS DLBCL) and ethnicity did not affect the overall survival outcomes in patients with DLBCL (Supplementary Table S2). Although the tumor location did not affect the overall survival of DLBCL, we reanalysed the effect of MYD88 L265P mutation on the overall survival outcomes of 394 DLBCL patients after excluding CNS DLBCL cases15. Nevertheless, there was no association between the MYD88 L265P mutation and overall survival outcomes (HR = 1.817; 95% CI: 0.599–5.515; p = 0.292, Q = 9.906, I 2 = 79.810). Finally, the sensitivity analysis showed that Kim et al.18 study influenced the pooled HR (Fig. 3), so the meta-analysis was performed again except for Kim et al.18 study. This pooling analysis showed that DLBCL patients with the MYD88 L265P mutation had low overall survival rates (HR = 3.244; 95% CI: 1.784–5.826; p < 0.001, Q = 0.068, I 2 = 0.000) (Supplementary Fig. S1).
Sensitivity analysis and publication bias
The sensitivity analysis showed that the pooled analyses of age18, sex17, 34, IPI18 and survival outcome18 according to MYD88 L256P mutation affected the pooled OR and HR, but the other analyses revealed that none of the studies affected the pooled OR with CIs (Fig. 4).
In the funnel plot and Egger regression test, there was no evidence of publication bias except for the meta-analysis for the association of MYD88 L265P mutation with the DLBCL subtypes (Supplementary Table S3) (Fig. 5).
Discussion
This pooled analysis showed that the tumor location of origin in DLBCL contributes to the prevalence difference of MYD88 L265P mutation. Moreover, the MYD88 L265P mutation is found in the ABC or non-GCB subtype more frequently than the GCB subtype of DLBCL.
We found that the incidence of MYD88 L265P mutation in DLBCL patients varies from tumor site to tumor site. CNS and testicular DLBCLs are present as extra-nodal localized masses and show inferior responses to current chemotherapy regimens8. Interestingly, Kraan et al. suggested that the MYD88 L265P mutation in DLBCL is associated with an immune-privileged anatomical compartment, such as the CNS or the testis21. Consistent with the previous result, this study showed that the MYD88 L265P mutation is most common in DLBCL at immunologically privileged sites.
This pooled analysis revealed that the MYD88 L265P mutation in DLBCL is significantly associated with the ABC or non-GCB subtype. ABC type DLBCL is characterized by chronic active B-cell receptor signaling and intrinsic activation of the NF-κB pathway, which can contribute to poor response to chemotherapy43. Initially, Ngo et al. claimed that GCB subtype has almost no MYD88 L265P mutation27. Since then, some studies have reported that the MYD88 L265P mutation occurs at a significantly higher frequency in ABC or non-GCB subtypes2, 21, 24, 30, 41. In contrast, other studies did not show a significant association between the MYD88 L265P mutation and the DLBCL molecular subtypes16, 18, 38.
The prognostic value of the MYD88 L265P mutation has been the matter of controversy. Some studies have reported that MYD88 L265P mutation is significantly associated with low survival rates11, 15, 31, 34. However, we did not reach a clear conclusion through the initial pooled analysis. Except for individual studies found not to be suitable for meta-analysis by sensitivity analysis, a second pool analysis revealed that the MYD88 L265P mutation was associated with a low survival rate and a high IPI risk group. Our results indicate that the MYD88 L265P mutation is significantly associated with patients older than 60 years, but not with gender and clinical stage. More studies are needed to demonstrate the negative prognostic effect of the MYD88 L265P mutation in DLBCL patients.
Some studies have mentioned other MYD88 mutations, such as S243N and S219C other than L265P2, 7, 21, 24, 27, 30, 34. In particular, Rovira et al. compared the clinical characteristics between L265P mutation and other MYD88 mutations34. However, there was not enough published data to perform a meta-analysis on this issue.
Targeting the MYD88 pathway in patients with DLBCL who do not respond well to anti-CD20 antibody therapy (rituximab) may be an attractive option. Interestingly, ABC type DLBCL with the MYD88 mutation often responds to Ibrutinib, a selective inhibitor of Bruton tyrosine kinase (BTK)44. Thus, the MYD88 mutation status of DLBCL may be a good predictor of chemotherapy in DLBCL patients.
There are several limitations to the current meta-analysis. First, we classified patients as white and Asian, but there may be discrepancies between our classification and the original data. Second, individual studies used different analytical methods and heterogeneous clinical samples. Third, the criteria for determining the molecular subtype of DLBCL differed somewhat between studies. Finally, the meta-analysis for overall survival could not be performed using the survival data from DLBCL patients completely excluding CNS and primary cutaneous DLBCL, leg type. We could not extract the survival HRs and CIs from nodal DLBCL from the published data because the previous studies presented only the survival HRs and CIs of DLBCL cases including some CNS and/or primary cutaneous DLBCLs. These limitations might affect the results of this pooled analysis.
Conclusion
This meta-analysis indicates that the MYD88 L265P mutation is a significant mutation in the DLBCL of the immune-privileged region and is significantly associated with the ABC or the non-GCB subtype of DLBCL. This mutation can be a powerful driver of high NF-κB activity, a characteristic of DLBCL’s ABC or non-GCB subtypes.
Methods
Data collection and selection criteria
We searched PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and EMBASE (www.embase.com) using the keywords “MYD88”, “lymphoma”, and “whole sequencing”. We also manually searched the reference lists of the identified articles. Duplicate data or overlapping articles were excluded by examining the authors’ names and affiliations. Original articles reporting cases of MYD88 L265P mutation published before September 2016 were included. When multiple articles were published by the same authors or institutions, the most recent or single informative article was selected. Articles lacking clinicopathologic data for meta-analysis, review articles without original data, conference abstracts, case reports, and articles that dealt with cell line or animal were excluded. There were no geographic or language restrictions. The selection process of the articles is shown in Fig. 6.
Data pooling and statistics
Meta-analysis was performed as previously described45. Briefly, effect sizes for each study were calculated by the prevalence rate and OR or HR and the corresponding 95% CI using the Mantel-Haenszel method or the Cohen method. The prevalence rates or ORs or HRs were combined using the random-effects model (DerSimonian-Laird method). The prevalence rates, ORs, or HRs were combined using the random-effects model (DerSimonian-Laird method). Statistical heterogeneity among studies was evaluated using the Cochrane Q test and I 2 statistics. The I 2 statistic refers to the percentage of variation across studies that is due to heterogeneity rather than chance and does not inherently depend on the number of studies considered [I 2 = 100% × (Q - df)/Q]. We clarified the cutoff of I 2 statistics for assignment of low (<25%), moderate (25–50%), and high (>50%) heterogeneities. If the I 2 value was more than 25%, subgroup analysis was done. Sensitivity analyses were performed to examine the influence of each study on the pooled prevalence rate and OR or HR by serially omitting an individual study and pooling the remaining studies. Publication bias was examined by funnel plots and Egger’s tests for the degree of asymmetry. If the P value is less than 0.1, it is assumed that a publication bias exists. The pooled analysis was performed using Comprehensive Meta-analysis Software version 2.0 (Biostat, Englewood, NJ, USA).
References
Jeelall, Y. S. & Horikawa, K. Oncogenic MYD88 mutation drives Toll pathway to lymphoma. Immunol Cell Biol 89, 659–660, doi:10.1038/icb.2011.31 (2011).
Bohers, E. et al. Targetable activating mutations are very frequent in GCB and ABC diffuse large B-cell lymphoma. Genes Chromosomes Cancer 53, 144–153, doi:10.1002/gcc.v53.2 (2014).
Bonzheim, I. et al. High frequency of MYD88 mutations in vitreoretinal B-cell lymphoma: a valuable tool to improve diagnostic yield of vitreous aspirates. Blood 126, 76–79, doi:10.1182/blood-2015-01-620518 (2015).
Braggio, E. et al. Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin Cancer Res 21, 3986–3994, doi:10.1158/1078-0432.CCR-14-2116 (2015).
Bruno, A. et al. Mutational analysis of primary central nervous system lymphoma. Oncotarget 5, 5065–5075, doi:10.18632/oncotarget.2080 (2014).
Caner, V. et al. MYD88 expression and L265P mutation in mature B-cell non-Hodgkin lymphomas. Genet Test Mol Biomarkers 19, 372–378, doi:10.1089/gtmb.2015.0041 (2015).
Cani, A. K. et al. Comprehensive genomic profiling of orbital and ocular adnexal lymphomas identifies frequent alterations in MYD88 and chromatin modifiers: new routes to targeted therapies. Mod Pathol 29, 685–697, doi:10.1038/modpathol.2016.79 (2016).
Chapuy, B. et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood 127, 869–881, doi:10.1182/blood-2015-10-673236 (2016).
Choi, J. W., Kim, Y., Lee, J. H. & Kim, Y. S. MYD88 expression and L265P mutation in diffuse large B-cell lymphoma. Hum Pathol 44, 1375–1381, doi:10.1016/j.humpath.2012.10.026 (2013).
Cox, M. C. et al. Clinicopathologic characterization of diffuse-large-B-cell lymphoma with an associated serum monoclonal IgM component. PLoS One 9, e93903, doi:10.1371/journal.pone.0093903 (2014).
Fernandez-Rodriguez, C. et al. MYD88 (L265P) mutation is an independent prognostic factor for outcome in patients with diffuse large B-cell lymphoma. Leukemia 28, 2104–2106, doi:10.1038/leu.2014.184 (2014).
Fukumura, K. et al. Genomic characterization of primary central nervous system lymphoma. Acta Neuropathol 131, 865–75, doi:10.1007/s00401-016-1536-2 (2016).
Gebauer, N. et al. Prevalence of targetable oncogenic mutations and genomic alterations in Epstein-Barr virus-associated diffuse large B-cell lymphoma of the elderly. Leuk Lymphoma 56, 1100–1106, doi:10.3109/10428194.2014.944522 (2015).
Gonzalez-Aguilar, A. et al. Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin Cancer Res 18, 5203–5211, doi:10.1158/1078-0432.CCR-12-0845 (2012).
Hattori, K. et al. MYD88 (L265P) mutation is associated with an unfavourable outcome of primary central nervous system lymphoma. Br J Haematol (2016).
Jimenez, C. et al. MYD88 L265P is a marker highly characteristic of, but not restricted to, Waldenström’s macroglobulinemia. Leukemia 27, 1722–1728, doi:10.1038/leu.2013.62 (2013).
Juskevicius, D. et al. Distinct genetic evolution patterns of relapsing diffuse large B-cell lymphoma revealed by genome-wide copy number aberration and targeted sequencing analysis. Leukemia (2016).
Kim, Y. et al. CD79B and MYD88 mutations in diffuse large B-cell lymphoma. Hum Pathol 455, 556–564, doi:10.1016/j.humpath.2013.10.023 (2014).
Knief, J. et al. Oncogenic mutations and chromosomal aberrations in primary extranodal diffuse large B-cell lymphomas of the thyroid–a study of 21 cases. J Clin Endocrinol Metab 100, 754–762, doi:10.1210/jc.2014-3250 (2015).
Koens, L. et al. Nuclear factor-κB pathway-activating gene aberrancies in primary cutaneous large B-cell lymphoma, leg type. J Invest Dermatol 134, 290–292, doi:10.1038/jid.2013.265 (2014).
Kraan, W. et al. High prevalence of oncogenic MYD88 and CD79B mutations in diffuse large B-cell lymphomas presenting at immune-privileged sites. Blood Cancer J 3, e139, doi:10.1038/bcj.2013.28 (2013).
Lohr, J. G. et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci USA 109, 3879–3884, doi:10.1073/pnas.1121343109 (2012).
Montesinos-Rongen, M. et al. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol 122, 791–792, doi:10.1007/s00401-011-0891-2 (2011).
Morin, R. D. et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 476, 298–303, doi:10.1038/nature10351 (2011).
Nagakita, K. et al. Clinicopathological features of 49 primary gastrointestinal diffuse large B-cell lymphoma cases; comparison with location, cell-of-origin, and frequency of MYD88 L265P. Pathol Int 66, 444–452, doi:10.1111/pin.2016.66.issue-8 (2016).
Nakamura, T. et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol 42, 279–290, doi:10.1111/nan.12259 (2016).
Ngo, V. N. et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 470, 115–119, doi:10.1038/nature09671 (2011).
Ogura, G. et al. MYD88 (L265P) mutation in malignant lymphoma using formalin-fixed, paraffin-embedded section. J Clin Exp Hematop 53, 175–177, doi:10.3960/jslrt.53.175 (2013).
Oishi, N. et al. High prevalence of the MYD88 mutation in testicular lymphoma: Immunohistochemical and genetic analyses. Pathol Int 65, 528–535, doi:10.1111/pin.12336 (2015).
Pasqualucci, L. et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet 43, 830–837, doi:10.1038/ng.892 (2011).
Pham-Ledard, A. et al. High frequency and clinical prognostic value of MYD88 L265P mutation in primary cutaneous diffuse large B-cell lymphoma, leg-type. JAMA Dermatol 150, 1173–1179, doi:10.1001/jamadermatol.2014.821 (2014).
Poulain, S. et al. Absence of CXCR4 mutations but high incidence of double mutant in CD79A/B and MYD88 in primary central nervous system lymphoma. Br J Haematol 170, 285–287, doi:10.1111/bjh.2015.170.issue-2 (2015).
Raja, H., Salomão, D. R., Viswanatha, D. S. & Pulido, J. S. Prevalence of MYD88 L265P mutation in histologically proven, diffuse large B-cell vitreoretinal lymphoma. Retina 36, 624–628, doi:10.1097/IAE.0000000000000996 (2016).
Rovira, J. et al. MYD88 L265P mutations, but no other variants, identify a subpopulation of DLBCL patients of activated B-cell origin, extranodal involvement, and poor Outcome. Clin Cancer Res 22, 2755–2764, doi:10.1158/1078-0432.CCR-15-1525 (2016).
Santos Gda, C. et al. Multiplex sequencing for EZH2, CD79B, and MYD88 mutations using archival cytospin preparations from B-cell non-Hodgkin lymphoma aspirates previously tested for MYC rearrangement and IGH/BCL2 translocation. Cancer Cytopathol 123, 413–420, doi:10.1002/cncy.21535 (2015).
Staiger, A. M. et al. Allele-specific PCR is a powerful tool for the detection of the MYD88 L265P mutation in diffuse large B cell lymphoma and decalcified bone marrow samples. Br J Haematol 171, 145–148, doi:10.1111/bjh.2015.171.issue-1 (2015).
Taniguchi, K. et al. Frequent MYD88 L265P and CD79B mutations in primary breast diffuse large B-cell lymphoma. Am J Surg Pathol 40, 324–334, doi:10.1097/PAS.0000000000000592 (2016).
Wang, C. Z. et al. Development of high-resolution melting analysis for the detection of the MYD88 L265P mutation. Clin Biochem 46, 385–387, doi:10.1016/j.clinbiochem.2012.11.007 (2013).
Xue, D., Lin, J. & Xiao, G. F. Detection of MYD88 mutation in lymphoma by PCR-high resolution melting curve analysis. Zhonghua Xue Ye Xue Za Zhi 34, 71–73 (2013).
Yamada, S., Ishida, Y., Matsuno, A. & Yamazaki, K. Primary diffuse large B-cell lymphomas of central nervous system exhibit remarkably high prevalence of oncogenic MYD88 and CD79B mutations. Leuk Lymphoma 56, 2141–2145, doi:10.3109/10428194.2014.979413 (2015).
Zhang, J. et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci USA 110, 1398–1403, doi:10.1073/pnas.1205299110 (2013).
Alizadeh, A. A. et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403, 503–511, doi:10.1038/35000501 (2000).
Compagno, M. et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature 459, 717–721, doi:10.1038/nature07968 (2009).
Wilson, W. H. et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 21, 922–926, doi:10.1038/nm.3884 (2015).
Lee, J. H., Choi, J. W. & Kim, Y. S. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. Br J Dermatol 164, 776–784, doi:10.1111/j.1365-2133.2010.10185.x (2011).
Acknowledgements
This work was supported by Mid-career Researcher Program through National Research Foundation of Korea (NRF) grant (grant no. 2016 R1A2B4012030) funded by the Ministry of Education, Science, and Technology.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the study and collected the data. J.H.L. and Y.S.K. performed the analyses and wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, JH., Jeong, H., Choi, JW. et al. Clinicopathologic significance of MYD88 L265P mutation in diffuse large B-cell lymphoma: a meta-analysis. Sci Rep 7, 1785 (2017). https://doi.org/10.1038/s41598-017-01998-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01998-5
This article is cited by
-
Deciphering the Prognostic Significance of MYD88 and CD79B Mutations in Diffuse Large B-Cell Lymphoma: Insights into Treatment Outcomes
Targeted Oncology (2024)
-
Clinicopathologic significance of MYD88 L265P mutation and expression of TLR4 and P-STAT3 in primary central nervous system diffuse large B-cell lymphomas
Brain Tumor Pathology (2021)
-
Genomic Alterations and MYD88MUT Variant Mapping in Patients with Diffuse Large B-Cell Lymphoma and Response to Ibrutinib
Targeted Oncology (2020)
-
Oncogenic MYD88 mutations in lymphoma: novel insights and therapeutic possibilities
Cancer Immunology, Immunotherapy (2018)
-
Patients with primary breast and primary female genital tract diffuse large B cell lymphoma have a high frequency of MYD88 and CD79B mutations
Annals of Hematology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.