Abstract
Plasma homocysteine (Hcy) levels may be associated with all-cause mortality risk. However, the results of this association are conflicting and the dose-response relationship between them has not been clearly defined. In this meta-analysis, we conducted a systematic literature search of the PubMed, Embase, Web of Science and Cochrane Library for the relevant articles dated up to February 2017. Pooled relative risks (RRs) and corresponding 95% confidence intervals (CIs) were calculated to evaluate the estimates, and the dose-response relationship was estimated using a restricted cubic spline model. Eleven prospective studies (4,110 deaths among 27,737 individuals) were included. The summary RR of all-cause mortality for the highest Hcy category vs. the lowest Hcy category was 1.80 (95% CI: 1.51, 2.14) with the random effects model. In dose-response meta-analysis, Hcy levels were significantly associated with all-cause mortality risk in a linear fashion (p nonlinearity = 0.255), and the risk of all-cause mortality increased by 33.6% for each 5 µmol/L increase in Hcy levels (RR = 1.336, 95% CI: 1.254–1.422, p < 0.001). Findings from this dose-response meta-analysis suggest that Hcy levels are linearly and positively associated with risk of all-cause mortality.
Similar content being viewed by others
Introduction
Homocysteine (Hcy), a sulphur-containing amino acid, is biosynthesized from methionine via a multi-step process1. A high level of homocysteine in the blood (hyperhomocysteinaemia) is correlated with a wide range of diseases, including cardiovascular disease, renal dysfunction, fractures, and cognitive impairment2,3,4,5.
Additionally, the association between Hcy levels and risk of all-cause mortality in human subjects has been explored, but the results are conflicting. In several follow-up studies, Hcy levels were reported to be associated with all-cause mortality6,7,8. For example, Vollset SE et al. reported that a 5 µmol/L increment of Hcy was correlated with an increase in all-cause mortality of 49%9. However, during an 11-year follow-up, Swart KM et al. found that elevated plasma Hcy levels were not related to the risk of mortality in older men10. Recently, a meta-analysis8 with six studies showed that elevated Hcy levels increased the risk of all-cause mortality in women (RR 1.74; 95% CI 1.24–2.44) and in the whole population (RR 1.93; 95% CI 1.54–2.43), but not in men (RR 1.87; 95% CI 0.64–5.50). Moreover, this meta-analysis with three studies found that the pooled RR of all-cause mortality was 1.27 for a 5-μmol/L increment in serum Hcy in the whole population, implying a possible potential dose-response. However, the small number of studies limits the statistical power and a further dose-response meta-analysis. Therefore, considering the above mentioned findings, it is necessary to assess the association between Hcy levels and risk of all-cause mortality with a larger dataset using a dose-response meta-analysis.
Results
Study characteristics
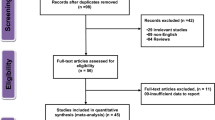
The study selection process is presented in Fig. 1. Initially, 5,651 potential studies were identified from the PubMed, Embase, Web of Science, and Cochrane Library databases; however, most of them were excluded according to the exclusion criterion. Finally, 11 eligible studies7, 9,10,11,12,13,14,15,16,17,18 with a total of 27,737 participants (4,110 deaths) were included. Of the included studies, 3 studies were performed in Asia, 2 in North America, and 6 in Europe. Sample sizes ranged from 215 to 7166, and the median follow-up time varied from 3.08 years to 11 years. Table 1 summarizes the general characteristics of the included studies.
Categorical meta-analysis
Our meta-analysis showed that elevated Hcy levels were associated with all-cause mortality risk. Further, the pooled relative risk (RR) (95% confidence interval [CI]) was 1.80 (1.51, 2.14) under the random-effects model with substantial heterogeneity among studies (I 2 = 64.8%, p H = 0.002, p z < 0.001) (Fig. 2). According to the funnel plot (Fig. 3a) and Egger’s test (p = 0.051), publication bias might exist, although the Begg’s test was not statistically significant (p = 0.119). Because of this, we conducted a trim and fill analysis that conservatively imputes hypothetical negative unpublished studies to mirror the positive studies that cause funnel plot asymmetry. The imputed studies showed a symmetrical funnel plot (Fig. 3b) and the pooled analysis continued to reveal a statistically significant association between Hcy levels and all-cause mortality risk (RR = 1.66, 95% CI: 1.39–1.97, p < 0.001).
Dose-response meta-analysis
Six studies7, 9, 11, 13, 14, 16 were included in the dose-response meta-analysis. The result showed a linear relationship between Hcy levels and all-cause mortality (p non-linearity = 0.255), by using a restricted cubic spline model. Furthermore, the linear model revealed an increased all-cause mortality risk of 33.6% for each 5 µmol/L increase in Hcy levels (RR = 1.336, 95% CI: 1.254–1.422, p < 0.001) (Fig. 4).
Subgroup and sensitivity analyses
In subgroup analyses of the association between Hcy levels and all-cause mortality, all subgroups revealed significant positive associations, except for the studies10, 16, 17 performed in men (RR = 1.44, 95% CI: 0.96–2.14, p = 0.077; Table 2). For studies that adjusted for smoking and diabetes, the pooled RRs (95% CIs) were 1.73 (1.44–2.08) and 1.69 (1.32–2.17), respectively, which were weaker than for those that did not adjust for these factors (RR = 2.2, 95% CI: 1.55–3.13; RR = 1.92, 95% CI: 1.58–2.33; respectively). For studies that adjusted for alcohol consumption, body mass index (BMI), physical activity, cardiovascular disease (CVD), and serum vitamin B12/ folate, the pooled RRs (95% CIs) were 1.91 (1.30–2.80), 1.91 (1.53–2.38), 2.10 (1.76–2.50), 1.94 (1.42–2.65), and 1.91 (1.30–2.80), respectively, which were stronger than for those that did not adjust for these factors (RR = 1.76, 95% CI: 1.46–2.12; RR = 1.72, 95% CI: 1.38–2.15; RR = 1.62, 95% CI: 1.35–1.93; RR = 1.62, 95% CI: 1.42–1.86; RR = 1.76, 95% CI: 1.46–2.12; respectively).
Sensitivity analysis revealed that no single study significantly changed the pooled results, which indicated that the results of our meta-analysis were robust and reliable (Fig. 5).
Discussion
It is important to explore whether Hcy levels were correlated with all-cause mortality. In the current meta-analysis, we identified that elevated Hcy levels were associated with the risk of all-cause mortality. Furthermore, the dose-response meta-analysis showed a linear association between Hcy levels and all-cause mortality risk, and each 5 µmol/L increment of Hcy corresponded to a 33.6% increase in risk of all-cause mortality.
Previously, a categorical meta-analysis, by including six studies, found that elevated Hcy levels were associated with all-cause mortality risk, and it found a significant dose-response association between them by including three studies8. Although the final conclusion was not significantly changed, there were some differences when comparing the two meta-analyses. First, by searching four databases (PubMed, Embase, Web of Science, and Cochrane Library), the present meta-analysis included more studies (11 studies for categorical meta-analysis and 6 studies for dose-response meta-analysis). Second, although dose response analysis was conducted in both studies, the methods and results were different. In the previous study, the dose response analysis was conducted based on 3 original articles that reported per 5 μmol/L increment in serum Hcy levels. In our study, the restricted cubic spline model was performed to estimate the dose-response relationship. Additionally, we found that Hcy levels were significantly associated with all-cause mortality risk in a linear fashion; this was not reported in the previous study. Third, compared with the previous study, we conducted adequate subgroup analyses, further investigated the potential inter-study heterogeneity, examined the robustness of primary results, and explored the influence of relative factors reported in previous studies.
In addition, Vollset SE et al., in a study including 4,766 individuals, reported that a 5-µmol/L increment of Hcy was correlated with a 49% increase in all-cause mortality, and, by using generalized additive logistic regression9, the increase in mortality was almost linear. Moreover, using restricted cubic spline, Wong YY et al. observed a graded association between Hcy and all-cause mortality17. Similarly, with 27,737 individuals included, the present study observed that elevated Hcy levels increased the risk of all-cause mortality in a linear fashion; this further indicated the association between Hcy levels and all-cause mortality. The potential biological mechanisms leading to the positive association between Hcy levels and risk of all-cause mortality are not well elucidated. It is likely that elevated Hcy levels may increase all-cause mortality risk by increasing the risk of major chronic diseases, including cardiovascular disease19, fracture20, cognitive decline21, and endothelial dysfunction22. Higher Hcy levels may cause platelet activation and thrombus formation23, imbalance between osteoblasts and osteoclasts24, hippocampal atrophy25, and endothelial dysfunction22, leading to cardiovascular disease, cognitive decline, fracture, endothelial dysfunction, which may eventually contribute to mortality. Further studies are needed to indicate the underlying biological mechanisms.
Hcy levels are typically higher in men than in women. Accordingly, several studies revealed that the association between Hcy levels and all-cause mortality might differ by sex. Swart KM et al.10 reported that elevated Hcy levels increased mortality risk in women only. A previous meta-analysis8 included two studies and identified that elevated Hcy levels increased the risk of all-cause mortality in women (RR 1.74; 95% CI 1.24–2.44); however, this positive association was not statistically significant in men (RR 1.87; 95% CI 0.64–5.50). Although we included one more study than the previous meta-analysis, we also did not observe the relationship between Hcy levels and mortality risk in men. Given that most previous studies on higher Hcy levels with mortality risk did not study sex differences, the number of included studies was still limited in the present meta-analysis (only three studies were included), and the results might not be robust enough to draw a conclusion. Thus, it is still unclear whether women or men with elevated Hcy had more mortality risk. Ongoing studies are needed.
Plasma Hcy levels are influenced by life-style and sociodemographic factors. For example, vitamin B and folate, as important cofactors, play an important role in Hcy metabolism. Inadequate dietary supply of vitamin B and folate may lead to hyperhomocysteinaemia26. Indeed, numerous studies have indicated that elevated Hcy levels were correlated with low levels of vitamin B and folate27, 28. In the present study, we identified that the pooled relative risk for the studies that adjusted for serum vitamin B12/ folate was 1.91 (95% CI: 1.30–2.80); this was stronger than that for studies that did not adjust for this factor (RR = 1.76, 95% CI: 1.46–2.12), suggesting that vitamin B/folate might reduce Hcy levels and thus decrease the risk of all-cause mortality. This subgroup analysis further demonstrated that increased Hcy levels were associated with all-cause mortality.
Although the present study revealed a positive association between Hcy levels and all-cause mortality, the findings should be considered with caution because of the moderate heterogeneity. There are several potential explanations for the observed inter-study heterogeneity. First, with subgroup analyses, we discovered that geographical location, duration of follow-up, sample size, fasting status, and NOS score contributed more or less to the potential heterogeneity, even though no specific factors were found. Second, lack of consistency in the categories of Hcy levels could also have contributed to inter-study differences in the strength of the observed associations. Third, the differences in adjusted confounding factors might have contributed to the heterogeneity to some extent.
A strength of this meta analysis was the prospective design (cohort studies and nested case-control studies were included), which should have greatly reduced the potential of selection bias. Numerous cohort studies with relatively large sample size and long follow-up enabled us to perform informative analysis in various subgroups of populations. In addition, the dose-response meta-analysis further provided a comprehensive description of the association of Hcy levels with all-cause mortality risk. Moreover, in this study, we conducted sensitivity analysis to reflect the influence of each study on the pooled estimates by removing one study at a time. We observed that the estimates did not change significantly, suggesting that the result of this meta-analysis was robust and reliable.
Despite our important findings, there were several limitations to our study. First, selection bias might have been introduced because of our relatively strict inclusion and exclusion criteria and failure to acquire complete data either from the original manuscript or from the authors; however, little evidence for such bias was observed. Second, the subjects in most of the studies were elderly; thus, whether the positive association between Hcy levels and all-cause mortality risk could be generalized to younger persons needs to be confirmed. Third, lack of consistency in the categories of Hcy levels might have contributed to inter-study differences that affected the strength of the identified associations. Fourth, the significant heterogeneity reduced the credibility of our results to an extent that the findings in current studies should be considered with caution. Fifth, publication bias was found according to the funnel plot and Egger’s test, although the trim and fill analysis did not change the general result (though the strength of the association was slightly attenuated), suggesting that the association is not an artefact of unpublished negative studies.
In summary, findings from this dose-response meta-analysis suggest elevated plasma Hcy levels are associated with linearly increasing risk of all-cause mortality. More in-depth studies are warranted to explore the roles of the biological mechanisms and public health interventions aimed at reducing Hcy levels.
Methods
The current meta-analysis was performed based on the published criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Search strategy
A computerized literature search was performed in PubMed, Embase, Web of Science, and Cochrane Library (dated up to February 2017) to collect articles regarding the correlation between Hcy levels and all-cause mortality. The following search terms were used: “Hcy” or “homocystine”, or “homocysteine”, or “L-Isomer homocysteine” or “2-amino-4-mercaptobutyric acid”, and “mortality” or “death”. Meanwhile, reference lists of relevant articles were also collected.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) prospective studies including cohort studies and nested case-control studies that explored the association of Hcy levels with all-cause mortality; (2) had reported estimates of RRs with 95% CIs or had presented data with which to calculate these; (3) the publication with longer follow-up and more applicable information was selected when multiple publications on the same study population were available; and (4) articles published in the English language.
The exclusion criteria were as follows: (1) the design was a retrospective study, animal study, meeting abstract, review article, editorial, case report, or other meta-analysis; (2) only unadjusted hazard ratio (HR), RR or odds ratio (OR) were reported, or raw data was unavailable for retrieval; and (3) subjects were in a highly selected disease group.
Data extraction and quality assessment
Two reviewers (Rui Fan and Aiping Zhang) independently extracted data, cross-checked the data, discussed all conflicts, and reached a consensus on all items. The following characteristics were extracted from each study: first author, year of publication, study location, design of study, duration of follow-up, sample size, age, sex, Hcy comparison, RRs, HRs or ORs with 95% CIs comparing the highest Hcy category with the lowest Hcy category, and adjustment for covariates. For studies that showed several multivariable-adjusted RRs, HRs or ORs, the risk estimates that adjusted for most confounding variables were selected.
The quality of the included studies was assessed independently by the same two reviewers mentioned above, according to the Newcastle–Ottawa Scale (NOS) criteria29. NOS scores ranged from 0 to 9, and a score of >7 indicated a high quality.
Statistical analysis
Categorical and dose-response meta-analyses were performed to assess the association between Hcy levels and all-cause mortality. Pooled RRs with their 95% CIs were used to evaluate the effect size for the association of Hcy levels with all-cause mortality. For studies that showed results separately for females or males, we pooled the results to obtain an overall estimate before combining the results with other studies.
The I 2 statistic was applied to quantify the inter-study differences; an I 2 value of 0% denoted no heterogeneity, 25% denoted low heterogeneity, 50% denoted moderate heterogeneity, and 75% denoted high heterogeneity30. The Q statistic was used to formally test for heterogeneity (p < 0.10 was considered statistically significant heterogeneity). The fixed-effects model was employed to calculate the RR when heterogeneity was found; otherwise, the random-effects model was applied. In this meta-analysis, subgroup analyses were further conducted to investigate the potential inter-study heterogeneity and examine the robustness of primary results31. We divided the subgroups as follows: geographical location (Asia16,17,18, North America11, 12, or Europe9, 10, 13,14,15, 32), sex (male10, 16, 17 or female10, 16), duration of follow-up (<7 years7, 9, 11, 14, 15, 17 or ≥7 years10, 12, 13, 16, 18), sample size (<100013,14,15 or ≥10007, 9,10,11,12, 16,17,18), fasting status (yes7, 11, 14, 15 or no12, 13, 16), and NOS score (≤77, 11, 17 or >79, 10, 12,13,14,15,16, 18). We also performed analyses stratified by whether the studies had adjusted for potential confounding factors, including smoking, alcohol consumption, BMI, physical activity, history of CVD, history of diabetes, and serum vitamin B12/folate.
For dose-response meta-analysis, the included studies should have reported the numbers of deaths and persons/person-years for at least 3 Hcy level categories and the mean or median values of the categories, or have presented the estimated midpoints of the categories if these were not shown in the studies. We assumed the open-ended categories were of the same amplitude as the adjacent categories when the highest categories were open-ended33. A 2-stage random-effects dose response meta-analysis was performed to examine the potential trend between Hcy levels and all-cause mortality34, 35. This was applied by modelling Hcy levels using a restricted cubic spline model with 4 knots (2 spline transformations) chosen at the 5th, 35th, 65th, and 95th percentiles of the exposure distribution36. In the first stage, by taking into account the correlation within each set of specific relative risks, a restricted cubic spline model was fitted to estimate the 2 study-level coefficients and the within-study covariance matrix34, 35. In the second stage, by pooling the study-specific coefficient estimates and variance/covariance matrices that had been obtained in the first stage, we derived the overall estimates with random effects37. To test the hypothesis that the coefficient associated with the second spline was different from 0, a p value for nonlinearity was calculated35.
We performed sensitivity analysis by removing one study at a time to calculate the overall homogeneity. Begg’s test,38 Egger’s test,39 and funnel plot were conducted to assess the presence of publication bias (p < 0.10 was considered statistically significant). The trim and fill analysis was also conducted to further evaluate the possible effect of publication bias in our meta-analysis40. This method considers the possibility of hypothetical “missing” studies that might exist, imputes their RRs, and recalculates a pooled RR that incorporates the hypothetical missing studies as though they actually existed. All analyses were performed using the software STATA version 12.0 (StataCorp LP, College Station, Texas, USA). Values of p < 0.05 were considered statistically significant.
References
Finkelstein, J. D. Pathways and regulation of homocysteine metabolism in mammals. Semin Thromb Hemost 26, 219–25 (2000).
Ganguly, P. & Alam, S. F. Role of homocysteine in the development of cardiovascular disease. Nutrition Journal 14, 6 (2015).
Chen, J. Y. et al. The association of leptin and homocysteine with renal function impairment in a population of Taiwanese adults. Clinical Nutrition 34, 943–50 (2015).
Kuroda, T., Tanaka, S., Saito, M., Shiraki, Y. & Shiraki, M. Plasma Level of Homocysteine Associated with Severe Vertebral Fracture in Postmenopausal Women. Calcified Tissue International 93, 269–275 (2013).
Smith, A. D. & Refsum, H. Homocysteine, B Vitamins, and Cognitive Impairment. Nutrition 36, 211 (2016).
Xiu, L. L. et al. Low and high homocysteine are associated with mortality independent of B group vitamins but interactive with cognitive status in a free-living elderly cohort. Nutrition Research 32, 928–939 (2012).
Waśkiewicz, A., Sygnowska, E. & Broda, G. Homocysteine concentration and the risk of death in the adult Polish population. Kardiologia Polska 70, 897–902 (2012).
Peng, H. Y., Man, C. F., Xu, J. & Fan, Y. Elevated homocysteine levels and risk of cardiovascular and all-cause mortality: a meta-analysis of prospective studies. Journal of Zhejiang Universityence B 16, 78 (2015).
Vollset, S. E. et al. Plasma total homocysteine and cardiovascular and noncardiovascular mortality: the Hordaland Homocysteine Study. Am J Clin Nutr 74, 130–6 (2001).
Swart, K. M., van Schoor, N. M., Blom, H. J., Smulders, Y. M. & Lips, P. Homocysteine and the risk of nursing home admission and mortality in older persons. European Journal of Clinical Nutrition 66, 188–95 (2012).
Acevedo, M., Pearce, G. L., Jacobsen, D. W., Minor, S. & Sprecher, D. L. Serum homocysteine levels and mortality in outpatients with or without coronary artery disease: an observational study. Am J Med 114, 685–8 (2003).
Bostom, A. G. et al. Nonfasting plasma total homocysteine levels and all-cause and cardiovascular disease mortality in elderly Framingham men and women. Arch Intern Med 159, 1077–80 (1999).
Dangour, A. D. et al. Plasma homocysteine, but not folate or vitamin B-12, predicts mortality in older people in the United Kingdom. J Nutr 138, 1121–8 (2008).
Gonzalez, S., Huerta, J. M., Fernandez, S., Patterson, A. M. & Lasheras, C. Homocysteine increases the risk of mortality in elderly individuals. Br J Nutr 97, 1138–43 (2007).
Hoogeveen, E. K. et al. Hyperhomocysteinemia increases risk of death, especially in type 2 diabetes: 5-year follow-up of the Hoorn Study. Circulation 101, 1506–11 (2000).
Kark, J. D. et al. Nonfasting plasma total homocysteine level and mortality in middle-aged and elderly men and women in Jerusalem. Ann Intern Med 131, 321–30 (1999).
Wong, Y. Y. et al. Homocysteine, frailty, and all-cause mortality in older men: the health in men study. J Gerontol A Biol Sci Med Sci 68, 590–8 (2013).
Xiu, L. L. et al. Low and high homocysteine are associated with mortality independent of B group vitamins but interactive with cognitive status in a free-living elderly cohort. Nutr Res 32, 928–39 (2012).
Bautista, L. E., Arenas, I. A., Peñuela, A. & Martı́Nez, L. X. Total plasma homocysteine level and risk of cardiovascular disease: A meta-analysis of prospective cohort studies. Journal of Clinical Epidemiology 55, 882–887 (2002).
Yang, J. et al. Homocysteine level and risk of fracture: A meta-analysis and systematic review. Bone 51, 376–382 (2012).
Ho, R. C. M. et al. Is high homocysteine level a risk factor for cognitive decline in elderly? A systematic review, meta-analysis, and meta-regression. American Journal of Geriatric Psychiatry Official Journal of the American Association for Geriatric Psychiatry 19, 607–617 (2011).
Celermajer, D. S. et al. Impaired endothelial function occurs in the systemic arteries of children with homozygous homocystinuria but not in their heterozygous parents. Journal of the American College of Cardiology 22, 854–8 (1993).
Dionisio, N., Jardín, I., Salido, G. M. & Rosado, J. A. Homocysteine, intracellular signaling and thrombotic disorders. Current Medicinal Chemistry 17, 3109–3119 (2010).
Herrmann, M. et al. The role of hyperhomocysteinemia as well as folate, vitamin B(6) and B(12) deficiencies in osteoporosis: a systematic review. Clin Chem Lab Med 45, 1621–32 (2007).
den Heijer, T. et al. Homocysteine and brain atrophy on MRI of non-demented elderly. Brain 126, 170–5 (2003).
Selhub, J. Homocysteine metabolism. Annu Rev Nutr 19, 217–46 (1999).
McRae, M. P. High-dose folic acid supplementation effects on endothelial function and blood pressure in hypertensive patients: a meta-analysis of randomized controlled clinical trials. J Chiropr Med 8, 15–24 (2009).
van Dijk, R. A., Rauwerda, J. A., Steyn, M., Twisk, J. W. & Stehouwer, C. D. Long-term homocysteine-lowering treatment with folic acid plus pyridoxine is associated with decreased blood pressure but not with improved brachial artery endothelium-dependent vasodilation or carotid artery stiffness: a 2-year, randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol 21, 2072–9 (2001).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–5 (2010).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–58 (2002).
Higgins, J., Thompson, S., Deeks, J. & Altman, D. Measuring inconsistency in meta-analyses. BMJ 327, 557–60 (2003).
Waskiewicz, A., Sygnowska, E. & Broda, G. Homocysteine concentration and the risk of death in the adult Polish population. Kardiol Pol 70, 897–902 (2012).
Il’Yasova, D., Hertz-Picciotto, I., Peters, U., Berlin, J. A. & Poole, C. Choice of Exposure Scores for Categorical Regression in Meta-Analysis: A Case Study of a Common Problem. Cancer Causes & Control 16, 383–8 (2005).
Orsini, N., Bellocco, R. & Greenland, S. Generalized least squares for trend estimation of summarized dose–response data. Stata Journal 6, 40–57 (2006).
Orsini, N., Li, R., Wolk, A., Khudyakov, P. & Spiegelman, D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. American Journal of Epidemiology 175, 66–73 (2012).
Desquilbet, L. & Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Statistics in Medicine 29, 1037–57 (2010).
Jackson, D., White, I. R. & Thompson, S. G. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Statistics in Medicine 29, 1282 (2010).
Begg, C. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–101 (1994).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–34 (1997).
Duval, S. & Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–63 (2000).
Acknowledgements
The research was supported by Ningbo Social Development Research Project (No. 2014C50051). We thank Dexing Hu and Lei Ma for valuable discussions.
Author information
Authors and Affiliations
Contributions
R.F. and F.D. designed the research. R.F. and A.P. reviewed the literatures. R.F. and A.P. collected the data. R.F. and A.P. analyzed the data. R.F. and F.D. wrote the paper. All authors critically reviewed the manuscript for important intellectual content and approved the final version.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fan, R., Zhang, A. & Zhong, F. Association between Homocysteine Levels and All-cause Mortality: A Dose-Response Meta-Analysis of Prospective Studies. Sci Rep 7, 4769 (2017). https://doi.org/10.1038/s41598-017-05205-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05205-3
This article is cited by
-
Associations of total homocysteine and kidney function with all-cause and cause-specific mortality in hypertensive patients: a mediation and joint analysis
Hypertension Research (2024)
-
A Proactive Intervention Study in Metabolic Syndrome High-Risk Populations Using Phenome-Based Actionable P4 Medicine Strategy
Phenomics (2024)
-
Homocysteine May Decrease Glucose Uptake and Alter the Akt/GSK3β/GLUT1 Signaling Pathway in Hippocampal Slices: Neuroprotective Effects of Rivastigmine and Ibuprofen
Molecular Neurobiology (2023)
-
Plasma homocysteine and macular thickness in older adults—the Rugao Longevity and Aging Study
Eye (2022)
-
Association of Homocysteine and Risks of Long-Term Cardiovascular Events and All-Cause Death among Older Patients with Obstructive Sleep Apnea: A Prospective Study
The Journal of nutrition, health and aging (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.