Abstract
MAP phosphatases (MKP)-1 acts as an important regulator of innate immune response through a mechanism of control and attention both MAPK and NF-κB molecules during bacterial infection. However, the regulatory role of MKP-1 in the interplay between MAPK and NFκB pathway molecules is still not fully understood. In present study, we showed a direct interactions of p38, ERK or IκBα with MKP-1, and demonstrated that MKP-1 was a pivotal feedback control for both MAP kinases and NF-κB pathway in response to S. aureus. In addition, we found that rolipram had anti-inflammatory activity and repressed IκBα activation induced by S. aureus via PKA-MKP-1 pathway. Our report also demonstrated that PKA-cα can directly bind to IκBα upon S. aureus stimulation, which influenced the downstream signaling of PKA pathway, including altered the expression of MKP-1. These results presented a novel mechanism of PKA and IκB pathway, which may be targeted for treating S. aureus infection.
Similar content being viewed by others
Introduction
Staphylococcus aureus is a Gram-positive bacterium that is responsible for the vast majority of life-threatening diseases, including serious skin and soft tissue infection, pneumonia, bacteremia, septic arthritis and sepsis1,2. S. aureus infection is capable of producing systemic cytokine responses. A wide range of inflammatory cytokines and chemokines are produced from blood monocytes and tissue macrophages3. It has become apparent that although peptidoglycan (PepG) and lipoteichoic acid (LTA), the major cell wall components of S. aureus are recognized by different class of pattern-recognition receptor (NOD or TLR2)4, they can trigger the same cascades of signaling events including the activation of the transcription factor NFκB and MAPK pathways, ultimately leading to the production a variety of pro-inflammatory cytokines, including TNFα, IL-1β and IL-65. TNFα is considered one of the key inflammatory mediators and acts as a host defense against bacterial infection. But, overproduction of TNFα also can cause septic shock, multiple organ dysfunction syndrome and inflammatory disorders6. Thus, both the induction and termination of pro-inflammatory cytokine production are all crucial for maintaining an appropriate defense during bacterial infection.
Mitogen-activated protein kinase phosphatases (MKPs) belong to a family of dual specificity protein phosphatases, which responsible for dephosphorylation of both phosphothreonine and phosphotyrosine residues7,8. MKP-1, the first defined member of MKPs9, acts as a crucial negative regulator of the inflammatory response in macrophages during bacterial infection10. Liu Y. group demonstrated that MKP-1 could limit the inflammatory reaction to S. aureus infection by inactivating MAPK signaling molecules10. Meanwhile, an increasing evidence suggested that the induction of MKP-1 could repress NFκB-dependent inflammatory genes expression11,12,13. NFκB may present a possible mechanism of action involved in MKP-1 related negative regulation of inflammatory responses. However, the regulatory role of MKP-1 in the interplay between MAPK and NFκB pathway molecules remains unclear.
The highly selective PDE4 inhibitor rolipram has been used for several years described for its anti-depression property14. In addition, anti-inflammatory action of rolipram has been appealed to treatments of autoimmune disorders, such as asthma, chronic obstructive lung disease (COPD)15. Several studies have indicated that rolipram could suppress TNFα synthesis and release in response to LPS in the monocytes16,17. Meanwhile, MKP-1 was shown to be a negative regulator of the inflammatory responses to different stimuli. It has also reported that MKP-1 was involved in the anti-inflammatory effect of rolipram17,18. Therefore, this study was designed to study the contribution of MKP-1 in rolipram associated anti-inflammatory activity by S. aureus stimulus.
In the present study, we reported that MKP-1 was a pivotal feedback regulator controlling both MAP kinases and NF-κB pathway. In addition, MKP-1 could directly interact with these molecules at different times, acted as a negative regulator of inflammatory response to S. aureus. In addition, we found that rolipram had anti-inflammatory activity through MKP-1 dependent mechanisms. Our results also represented that PKA-cα can directly bind to IκBα in response to S. aureus, which influenced the downstream signaling of PKA pathway, including altered the expression of MKP-1. These results brought a novel mechanism between PKA and IκB pathway, which may be targeted for treating S. aureus infection.
Results
MKPs expressions were enhanced and more robust induction of MKP-1 by S. aureus stimulus
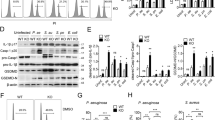
To determine whether MKP family played a role in regulating of S. aureus induced immune response, we first examined the expressions of three distinct subgroups of MKPs: DUSP1/MKP-1, DUSP6/MKP-3 and DUSP10/MKP-5. In response to S. aureus, the induction of MKP-1 became evident at 15 minutes, greatly up-regulated from 30 to 120 minutes, and returned to the basal levels at 240 minutes in Raw264.7 cells (Fig. 1a,b). Western-blot results also showed that S. aureus increased MKP-3 and MKP-5 expression from 15 to 60 minutes, the peak was at 30 minutes, as shown in Fig. 1a,b. Compared with MKP-3 and MKP-5, MKP-1 was more robust production from 15 to 120 minutes, which indicated that MKP-1 may play a significant role in the innate response by S. aureus stimulus. Meanwhile, we assessed the expression of Mkp-1 at mRNA level in Raw264.7 cells by q-PCR analysis. Similarly, Mkp-1 induction was increased at transcription level by S. aureus stimulus (Fig. 1c).
Expressions of MKPs and related signaling molecules induced by S. aureus in Raw264.7 cells. (a–c) Raw264.7 cells stimulated with S. aureus (10 M.O.I.) or control. (a,b) Cells were harvested for protein after 0, 15, 30, 60 and 120 minutes respectively, and cell lysates were subjected to western blot analyses for MKP-1, MKP-3, MKP-5 and β-actin. Immunoblots were scanned, the intensities of bands were quantified by the Image program. Data were normalized to the control, and the ratios were expressed as fold changes with respect to the control samples. (c) Cells were harvested for RNA after 60, 120 and 240 minutes, and real-time PCR was carried out using primers specific to Mkp-1 and β-actin. (d–k) Raw264.7 cells were incubated with U 0126 (10 μM), SB 203580 (10 μM) and Bay 11-7082 (10 μM) as indicated for 30 minutes prior to stimulation with S. aureus (10 M.O.I.) or control. (d–j) Cells were harvested for 15 to 30 minutes, western blot analyses of MKP-1, phospho-ERK1/2, phospho-p38, phospho-IκBα and β-actin were carried out. (k) Cells were harvested for 60 to 120 minutes, and real-time PCR was carried out using primers specific to Mkp-1 and β-actin. *p < 0.05.
Increased MKP-1 expression by S. aureus stimulus was suppressed by ERK, p38 and IκBα inhibitors. To explore the signaling cascade of MKP-1 expression in S. aureus activated Raw264.7 cells, we utilized U 0126 (MEK-ERK1/2 inhibitor), SB 203580 (p38 inhibitor) and Bay 11-7082 (IκBα inhibitor) to assess MKP-1 expression. Since mRNA and protein analyses for MKP-1 revealed that a significant increase was observed from 15 to 120 minutes after S. aureus stimulus, this period was used to investigate the effects of these pharmacological inhibitors on the induction of MKP-1 (Fig. 1d–k). Treatments of Raw264.7 cells with U 0126 and SB 203580 (10 μM, pretreatment for 30 minutes) were capable of inhibiting MKP-1 production induced by S. aureus at protein level (Fig. 1d,e,g,h,j). Compared with U 0126 and SB 203580, Bay 11-7082 (10 μM, pretreatment for 30 minutes) also had apparent effect on MKP-1 expression upon S. aureus stimulation (Fig. 1f–j). Meanwhile, the effects of these pharmacological inhibitors on Mkp-1 expression at transcription level were also predominant (Fig. 1k). Pretreatments of ERK and p38 inhibitors (10 μM for 30 minutes) suppressed the expression of Mkp-1 stimulated by S. aureus at 60 and 120 minutes respectively (about 0.5 and 0.6 fold). In addition, Bay 11-7082 (10 μM, pretreated for 30 minutes) was capable of inhibiting Mkp-1 levels from 60 to 120 minutes in response to S. aureus stimulation (about 0.3 to 0.4 fold). The effects of these pharmacological inhibitors on MKP-1 expression in Raw264.7 cells revealed that ERK, p38 and IκBα signaling cascades were all involved in MKP-1 synthesis by S. aureus stimulus.
MKP-1 was required for attenuation of TNFα production induced by S. aureus
To assess the innate immune response of Raw264.7 cells to S. aureus infection, we then determined the effect of S. aureus on the pro-inflammatory cytokine-TNFα at transcription level. S. aureus induced production of TNFα via the time-dependent manner (Fig. 2a). Maximal TNFα mRNA level was achieved at 60 minutes after stimulation of S. aureus, which gradually decreased from 120 to 240 minutes. Our previous study demonstrated that MKP-1 could inhibit TNFα production in the macrophages by LPS incubation17, we asked whether Mkp-1 could attenuate the production of TNFα by S. aureus stimulus. As shown in Fig. 2b, the expression of TNFα was increased significantly by siRNA-Mkp-1 approach. These results indicated that the loss of Mkp-1 function increased production of TNFα induced by S. aureus in Raw264.7 cells.
Effect of Mkp-1 target siRNA on S. aureus induced TNFα mRNA synthesis and p38, ERK, IκBα phosphorylations in Raw264.7 cells. (a) Raw264.7 cells were stimulated with S. aureus (10 M.O.I.) or control for 60, 120 and 240 minutes. Cells were harvested, real-time PCR was carried out using primers specific to TNFα and β-actin. (b) Raw264.7 cells stably harboring the Mkp-1 siRNA and Raw264.7 cells were simulated with S. aureus (10 M.O.I.) or control for different time, real-time PCR was performed for TNFα and β-actin. (c) Raw264.7 Mkp-1 siRNA and Raw264.7 cells were stimulated with S. aureus (10 M.O.I.) or control as indicated for 15, 30, 60, 120 and 240 minutes. Cell lysates were subjected to western blot analyses for MKP-1, phospho-ERK1/2 (ERK2), phospho-p38 (p38) and phospho-IκBα (IκBα) and β-actin. (d–i) Data were normalized to the control and expressed as the percentage of maximum activation of fold change of stimulation. *p < 0.05.
MKP-1 attenuated TNFα production induced by S. aureus via suppression of ERK, p38 and IκBα activations
Some studies have shown that S. aureus or their bacterial components can trigger a cascade of signaling events to activate the NFκB and MAPK pathways, leading to production of a variety of pro-inflammatory cytokines, such as TNFα4,5,19. To investigate the activations of ERK, p38 and IκBα on TNFα biosynthesis induced by S. aureus, we utilized the pharmacological inhibitors of these signaling molecules to assess TNFα production. As shown in Supplemental Fig. S1, ERK, p38 or IκBα inhibitor (U 0126, SB 203580 or Bay 11-7082, pretreatment for 30 minutes) all decreased the expression of TNFα at transcription level at 60 and/or 120 minutes after S. aureus stimulus (about 0.1 to 0.5 fold). These data demonstrated that ERK, p38 and IκBα molecules were all able to regulate S. aureus-mediated TNFα production in Raw264.7 cells.
To further explore the role of S. aureus induced MKP-1 in the repression of inflammatory molecules, we assessed the expressions of MKP-1 and related signaling molecules in Mkp-1 knockdown Raw264.7 cells. In Raw264.7 WT cells, S. aureus stimulation resulted in an increase in MKP-1 protein level, which temporally coincided with the inactivations of ERK1/2 and p38. No MKP-1 protein was detected in Mkp-1 siRNA cells when stimulated by S. aureus (Fig. 2c,d). Gene silencing of Mkp-1 by RNAi in Raw264.7 cells exhibited a substantial increase and prolonged (from 15 to 60 minutes) ERK1/2 activation compared with WT cells (Fig. 2c,e,f). In addition to the total ERK2, our western-blot analyses revealed an immunoreactive band of slightly higher molecular weight. The manufacturer’s data sheets of ERK2 (Santa Cruz Biotechnology, USA) suggested this band should be ERK1, we would not comment further on this band. Consistent with the sustained ERK1/2 activation in Mkp-1 siRNA cells, S. aureus stimulation resulted in an increased p38 activation (at 15 and 30 minutes) in Mkp-1 siRNA cells. However, our results showed that there was no difference in duration of p38 activation between 60 and 240 minutes by S. aureus stimulus (Fig. 2c,g). Interestingly, MKP-1 was also involved in attenuation of IκBα activation induced by S. aureus. Maximal expression of phospho-IκBα was detected at 30 minutes after S. aureus stimulation and gradually returned to the basal level till 120 minutes. Mkp-1 targeting siRNA produced an increased, earlier and prolonged (from 15 to 240 minutes) IκBα activation, but the total IκBα protein levels were not changed when treated with S. aureus (Fig. 2c,h,i). Meanwhile, our previous data demonstrated that Mkp-1 targeting siRNA had a significant increase in the production of TNFα. In addition, ERK, p38 and IκBα molecules were all involved in regulating S. aureus-mediated TNFα production in Raw264.7 cells. Considered together, it was possible that S. aureus induced MKP-1 could inhibit IκBα, ERK and p38 mediated cytokines production by suppression of their activations.
PKA pathway was involved in MKP-1 production induced by S. aureus
Several lines of evidence indicated that PKA pathway was involved in the innate immune response and the cytokine release when stimulated with the PepG and LTA, the pure cell wall constituents of S. aureus 20,21. Meanwhile, some studies have shown that PKA catalytic subunit (PKA-c) was involved in the inflammation during bacterial infection in macrophages22,23,24,25. Then we explored the effect of S. aureus on PKA-cα (one catalytic isoform of PKA-c) expression in Raw264.7 cells. As shown in Fig. 3a,b, S. aureus treatment induced a substantial high level expression of PKA-cα, which greatly up-regulated at 30 minutes, reached a maximum value at 60 minutes and decreased a little at 120 minutes.
Involvement of PKA-c activation in S. aureus induced MKP-1 production. (a,b) Raw264.7 cells were either not stimulated or stimulated with S. aureus (10 M.O.I.). Cells were harvested for protein after 0, 15, 30, 60 and 120 minutes, and cell lysates were subjected to western blot analyses for PKA-cα and β-actin. (c) Raw264.7 cells were incubated with KT-5720 (0.1~5 μM) as indicated for 30 minutes prior to stimulation with S. aureus (10 M.O.I.) for 60 minutes. Cells were harvested for RNA, and real-time PCR was carried out using primers specific to Mkp-1 and β-actin. (d–h) Raw264.7 cells were incubated with KT-5720 (1 μM) as indicated for 30 minutes prior to stimulation with S. aureus (10 M.O.I.). Cell lysates were subjected to western blot analyses for MKP-1, phospho-CREB, phospho-IκBα, phospho-p38 and phospho-ERK1/2 for indicated time. Immunoblots were scanned, the intensities of bands were quantified by the Image program. Data were normalized to the control, and the ratio was expressed as fold change with respect to the control sample or as the percentage of maximum activation of stimulation. *p < 0.05.
Some studies have reported that MKP-1 was involved in the PKA signaling when treated with rolipram17,18, we hypothesized that S. aureus induced MKP-1 could be regulated by PKA pathway. We took advantage of a specific inhibitor for PKA, KT-5720 and assessed MKP-1 expression by S. aureus stimulus. Since the peak of S. aureus induced Mkp-1 mRNA expression occurred at 60 minutes, this period was used to study the effect of KT-5720 on the induction of Mkp-1. As shown in Fig. 3c, when pre-treated Raw264.7 cells with KT-5720 (0.1~5 μM, 30 minutes) could inhibit S. aureus induced Mkp-1 transcription via the dose-dependent manner. KT-5720 (1 μM and 5 μM) suppressed S. aureus induced Mkp-1 production at 60 minutes (about 0.3 fold).
CREB is known to be one of the major downstream molecule in PKA pathway. PKA is able to activate CREB by phosphorylation at Ser133. CREB (Ser133) has been used to monitor the activation of PKA in numerous studies26. S. aureus stimulation induced a transient activation of CREB in Raw264.7 cells (up-regulated at 30 minutes, decreased at 60 minutes, returned to the basal level at 120 minutes, as shown in Fig. 3d). KT-5720 (1 μM, 30 minutes) attenuated S. aureus induced CREB (Ser133) phosphorylation at 60 minutes. In addition, S. aureus induced MKP-1 expression was also suppressed by KT-5720 (Fig. 3d–f) at 60 minutes (0.6 fold) and 120 minutes (0.3 fold). Taken together, it suggested that S. aureus induced MKP-1 production could be regulated by PKA pathway in Raw264.7 cells.
Rolipram repressed TNFα production induced by S. aureus as well as the activation of related signaling molecules
Among the large phosphodiesterases (PDEs) family, PDE4 is mainly expressed in neutrophils, macrophages and T cells. An increasing number of data now indicated that the PDE4 inhibitor had the negative modulatory effects on the inflammatory responses, including inflammatory cells activation and cytokines release15,16,17,18. We thus utilized PDE4-specific inhibitor, rolipram to pre-treat Raw264.7 cells, and then assessed the production of TNFα induced by S. aureus stimulus. Rolipram (10 μM, pretreated for 30 minutes) could inhibit S. aureus induced TNFα expression at the mRNA level in Raw264.7 cells (Fig. 4a,b). Then we explored the effects of rolipram on TNFα expression related signaling molecules. As shown in Fig. 4c,e,f, phosphorylations of p38 and IκBα were increased in response to S. aureus stimulus and they were reduced obviously by rolipram. Compared with p38 and IκBα, rolipram had little effect on the activation of ERK1/2 (Fig. 4c,g,h). The inhibitory effects of rolipram on TNFα production and related signaling molecule activations indicated that rolipram could inhibit inflammatory gene expression and inflammation induced by S. aureus.
Effect of rolipram on TNFα production and related signaling molecules activation induced by S. aureus in Raw264.7 cells. Raw264.7 cells were incubated with rolipram (10 μM) for 30 minutes prior to stimulation with S. aureus (10 M.O.I.) or control. (a,b) Cells were harvested for 60, 120 and 240 minutes. Relative quantity and percentage of TNF mRNA expression was measured by real-time PCR analysis. (c) Cells were harvested for 15, 30, 60 and 120 minutes, MKP-1, phospho-IκBα, phospho-p38 and phospho-ERK1/2 were measured by western-blot analyses. (d–h) The chemiluminescent signal was normalized to the control and expressed as the percentage of maximum activation of stimulation. *p < 0.05.
Rolipram enhanced MKP-1 expression in resting and activated Raw264.7 cells
To evaluate the contribution of MKP-1 in rolipram-mediated anti-inflammatory effects, we assessed MKP-1 expression in Raw264.7 cells after treated with rolipram. As shown in Fig. 4c,d, MKP-1 expression was increased by S. aureus stimulus and it was further enhanced in the presence of rolipram in Raw264.7 cells. In addition, induction of MKP-1 was increased obviously at both transcription and translation levels by administration of rolipram alone in the Raw264.7 cells (Figs 4c,d and 5a–c). Our previous data demonstrated that MKP-1 could be a major feedback regulator of innate immune response and suppress ERK, p38 and IκBα mediated TNFα production. Taken together, these data indicated that rolipram could enhance MKP-1 expression and MKP-1 may be involved in the anti-inflammatory effects of rolipram by S. aureus stimulus.
Effects of rolipram on MKP-1 and PKA-cα expression in Raw264.7 cells. (a) Raw264.7 cells were treated with rolipram (10 μM) for 60, 120 and 240 minutes. Cells were harvested for RNA, and real-time PCR was carried out using primers specific to Mkp-1 and β-actin. Cells were treated with rolipram (10 μM). for 0, 15, 30, 60 and 120 minutes, and cell lysates were subjected to western blot analyses for MKP-1 (b,c), PKA-cα (d,e) and β-actin. Immunoblots were scanned, the intensities of bands were quantified by the image program. Data were normalized to the control, and the ratio was expressed as fold change with respect to the control sample. *p<0.05.
Rolipram up-regulated MKP-1 expression via cAMP-PKA dependent signaling pathway
It has been demonstrated that PDE4 is a cAMP-specific PDE which is expressed in the inflammatory cells, inhibition of PDE4 by rolipram can lead to an elevation of endogenous cAMP levels and down-regulation of the inflammatory response18. To investigate the role of cAMP in rolipram induced anti-inflammatory effects, we checked endogenous cAMP levels in our experiments by rolipram incubation. We also challenged the Raw264.7 cells with the cAMP-elevating agents such as forskolin (activator of adenylyl cyclase) and 8-Bromo-cAMP (activator of protein kinase A) as the positive control. Compared with forskolin and 8-Bromo-cAMP, rolipram displayed modest but predominant effects on cAMP levels in Raw264.7 cells (Supplemental Fig. S2).
Some studies have shown that cAMP-dependent suppression of TNFα production is mediated via PKA-cα in macrophages by LPS stimulation22,23,24,25. To further investigate the role of PKA-cα in rolipram induced anti-inflammatory effect, we assessed the expression of PKA-cα in Raw264.7 cells after treating with rolipram. As shown in Fig. 5d,e, treatment with rolipram substantially increased PKA-cα expression in the time-dependent manner, which suggested that the involvement of cAMP-PKA signaling in rolipram mediated anti-inflammatory effects.
Since there is convince evidence that MKP-1 expression could be regulated by cAMP-PKA pathway17,18,27, we then sought to determine the effect of cAMP-PKA pathway on MKP-1 expression induced by S. aureus in Raw264.7 cells. We utilized the cAMP-elevating agents to assess the expression of MKP-1 in Raw264.7 cells by S. aureus stimulus. As shown in Fig. 6a,b, the expression of MKP-1 was significant induced by forskolin and 8-Bromo-cAMP alone or in combination with S. aureus in Raw264.7 cells. Similar to the cAMP-elevating agents, S. aureus-stimulated MKP-1 expression was increased obviously by rolipram, and it was further enhanced in the presence of rolipram in Raw264.7 cells. Taken together, these data indicated that PDE4 inhibitor rolipram could enhance the expression of MKP-1, and at least partly, by cAMP-PKA dependent pathway.
Effects of rolipram, foskolin and 8-Bromo-cAMP on MKP-1 expression and p38, ERK, IκBα phosphorylations induced by S. aureus in Raw264.7 cells. Raw264.7 cells were pre-incubated with rolipram (10 μM), foskolin (10 μM) and 8-Bromo-cAMP (250 μM) for 30 minutes prior to stimulation with S. aureus (10 M.O.I.) or control. (a,d) MKP-1/phospho-IκBα (30, 60 minutes) and phospho-p38/phospho-ERK1/2 (15, 30 minutes) were measured by western-blot analyses. The chemiluminescent signal was normalized to the control and expressed as the percentage of maximum activation of stimulation (b,c,e,f). *p < 0.05.
Rolipram repressed IκBα activation induced by S. aureus via cAMP-PKA pathway
Our previous data showed that the anti-inflammatory effect of rolipram on S. aureus induced inflammation was mediated by MKP-1 via cAMP-PKA pathway. We also found that MKP-1 was required for attenuation of inflammation induced by S. aureus via suppression of IκBα, p38 and ERK activations. We hypothesized that IκBα, p38 and ERK molecules may involve in attenuation of inflammation caused by rolipram via cAMP-PKA pathway. In order to verify the hypothesis, we utilized the rolipram and cAMP-elevating agents to assess the activated forms of IκBα, ERK and p38 in Raw264.7 cells by S. aureus stimulus. As shown in Fig. 6a,c, treatments of Raw264.7 cells with rolipram, forskolin or 8-Bromo-cAMP were all capable of inhibiting IκBα activation induced by S. aureus at 30 and 60 minutes. These agents also had obvious effects on p38 activation induced by S. aureus, resulting in ~40% reduction in p38 phosphorylation at 30 minutes (Fig. 6d,e). Meanwhile, prior to incubation with rolipram and other agents revealed modest effect on ERK activation (Fig. 6d,f). Therefore, these data demonstrated that IκBα and p38 molecules could be regulated by increasing intracellular cAMP concentration in Raw264.7 cells.
Because PKA represents the major downstream signaling effector of cAMP, we continued by investigating the effect of PKA inhibitor (KT 5720) on S. aureus induced activations of IκBα, p38 and ERK in Raw264.7 cells. As shown in Fig. 3g,h, treatment of Raw264.7 cells with KT-5720 (1 μM, pretreated 30 minutes) markedly enhanced the activation of IκBα by S. aureus stimulus. Meanwhile, in response to S. aureus, administration of KT-5720 had little effects on p38 and ERK phosphorylations. Furthermore, our previous results showed that KT-5720 could inhibit S. aureus induced MKP-1 production. Taken together, these data indicated that the interaction between MKP-1 and IκBα by S. aureus stimulus could be regulated via cAMP-PKA pathway.
Interactions of ERK, p38 and IκBα with MKP-1
It has been shown that MKP-1 expression can be up-regulated through the interaction with p38 and ERK molecules using biochemical methods28,29. Our previous studies also suggested that knockdown of Mkp-1 in Raw264.7 cells resulted in a substantial increase of p38 and ERK phosphorylations by S. aureus stimulus. We explored the possibility of a direct interaction between MKP-1 and p38 in Raw264.7 cells by S. aureus stimulus. After stimulating the Raw264.7 cells by S. aureus at different times, the immunoprecipitation was performed using antibody against MKP-1 (Fig. 7a). The precipitates were fractionated by SDS-PAGE and immunoblotted with antibody against p38. In the resting cells, p38 was not linked to MKP-1 (Fig. 7a, lane 1). After stimulated with S. aureus, p38 was efficiently precipitated with MKP-1 at 15 minutes, then this interaction was decreased (Fig. 7a, lane 2–5). We also assessed the ability of MKP-1 to bind ERK directly. As shown in Fig. 7a, ERK was sufficiently detected in precipitates of MKP-1 at 30 and 60 minutes (lane 3–4). It was not the case in the resting cells, indicating that there was a specific affinity between MKP-1 and ERK after stimulated with S. aureus.
Interactions of p38, ERK, IKBα with MKP-1 and PKA-cα, directly associated with IKBα. Raw264.7 cells were either not stimulated or stimulated with S. aureus (10 M.O.I.). Cells were harvested for protein after 0, 15, 30, 60 and 120 minutes respectively. (a) MKP-1 was immunoprecipitated (IP) from cell lysates and analyzed by western blotting for presence of p38, ERK, IKBα and MKP-1. (b) PKA-cα was IP from cell lysates and analyzed by western blotting for presence of IKBα and PKA-cα. (c) Cell lysates were subjected to western blot analyses for phospho-CREB and β-actin.
Our previous data demonstrated that IκBα activation was markedly enhanced in siRNA-Mkp-1 Raw264.7 cells by S. aureus stimulus. We hypothesized that MKP-1 may exert its effect through direct interaction with IκBα in Raw264.7 cells. We thus tested our hypothesis by assessing the interaction between MKP-1 and IκBα. Immunoprecipitation in our study suggested that IκBα could precipitate with MKP-1 at 120 minutes after S. aureus stimulation (Fig. 7a, lane 5). Interestingly, although direct interactions of p38, ERK or IκBα with MKP-1 were detected in Raw264.7 cells stimulated by S. aureus, they exhibited different peak time when linking to MKP-1. Taken together, these results indicated that MKP-1 could modulate MAPK pathway and NFκB cascade by direct interactions with these molecules (p38, ERK and IκBα) at different times. Thus, MKP-1 could be acted as a negative regulator for S. aureus induced inflammatory response.
PKAc, directly associates with IKBα
The data above showed that MKP-1 could control IκBα by direct interaction and thus it might be modulated by cAMP-PKA pathway. In addition, some other groups demonstrated that PKA-cα, but not PKA-cβ, PKA-cγ nor any of R subunit could bind specifically to the cytosolic IκBα and regulated the activity of NFκB30. This interaction was further confirmed by another study on platelet activation by thrombin and collagen stimulation31. We then explored a direct interaction between PKA-cα and IκBα by S. aureus stimulus. As the procedures described above, experiments using the antibody against PKA-cα, the immunoprecipitates were fractioned and immunoblotted with antibody against IκBα. IκBα was detected at the resting cells (Fig. 7b, lane 1). This result was consisted with the studies in other cells30,31. After stimulated by S. aureus for 15 minutes, the binding between PKA-cα and IκBα was remarkably decreased, almost could not be detected (Fig. 7b, lane 2), which suggested stimulation of Raw264.7 cells with S. aureus activated NFκB, at least in part through IκBα-dependent pathway, phosphorylated IκBα, disrupted the PKAcα-IκBα complex, and released the free, active PKA-cα. At the same time, CREB, the major downstream molecule in PKA pathway, was greatly activated by phosphorylation at Ser133 (Fig. 7c, lane 2). Additionally, IκBα was re-detected at 30 minutes by S. aureus, more efficiently precipitated at 60 minutes, and decreased a little at 120 minutes. These data indicated that the PKAcα-IκBα complex re-constituted by S. aureus stimulation from 30 to 120 minutes. Consistent with these results, PKA-cα expression was greatly up-reregulated in this period (Figs 6a and 7b, lane 3–5). To further explored whether this interaction would modulate the activity of PKA, we assessed the expression of CREB (Ser133). In contrast, the phosphorylation at Ser133 of CREB was sharply decreased at 60 minutes, returned to the basal level at 120 minutes by S. aureus in Raw264.7 cells (Fig. 7c, lane 4–5). These results demonstrated that IκBα associated with PKA-cα suppressed the catalytic activity of PKA, and influenced the downstream signaling of PKA pathway. Our report may present a novel mechanism on PKA and IκB pathway in an auto-regulatory feedback manner by S. aureus stimulus in Raw264.7 cells.
Discussion
The present study demonstrated that MKP-1 acted as a negative regulator of inflammatory response to S. aureus via controlling both MAP kinases and NF-κB molecules. In addition, we found that the anti-inflammatory effect of rolipram on S. aureus induced inflammation was mediated by MKP-1 via cAMP-PKA pathway. Furthermore, we uncovered the relationship between PKA-cα and IκBα in response to S. aureus, and our study provided a novel mechanism between MKP-1 and anti-inflammatory effect of rolipram by S. aureus stimulus in Raw264.7 cells.
A major function of the innate immune cells during microbial infection is to detect the pathogen-associated molecular patterns through their specialized receptors and trigger an evolutionarily signaling cascades, which in turn induce the production of pro-inflammatory cytokines and chemokines4,5,19. On the other hand, a variety of negative regulator could modulate the strength/duration of the signals and control the production of inflammatory cytokines6. MKPs has been shown to act as a crucial negative regulator of the inflammatory response in macrophages during different stimuli10. In our study, MKP-1 was robust induced by S. aureus stimulus. In addition, the production of TNFα was greatly increased when using the Mkp-1 targeting siRNA approach, which implicated that MKP-1 could play an important role in response to S. aureus stimulation. Previous studies have demonstrated that MKP-1 could be rapidly up-regulated and appeared to exhibit a broad substrate specificity in some infection status9, we then assessed the effects of different pharmacological inhibitors on MKP-1 expression induced by S. aureus stimulus in Raw264.7 cells. In present study, S. aureus induced a transient activation of MAPK and NFκB molecules. Treatments of Raw264.7 cells with the ERK, p38 and IκBα inhibitors sharply attenuated S. aureus induced MKP-1 production at both mRNA and protein levels. Meanwhile, knockdown of Mkp-1 in Raw264.7 cells exhibited the substantial increase and/or prolonged the p38, ERK and IκBα activations compared with WT cells. Furthermore, the immunoprecipitation experiments showed that a direct interactions of p38, ERK or IκBα with MKP-1 at different times in Raw264.7 cells by S. aureus stimulus. Our data suggested that MKP-1 would be a pivotal feedback control both MAP kinases and NF-κB pathway in response to S. aureus. This finding was consistent with previous studies indicating that MAPK and NFκB molecules were all associated with MKP-1 dependent negative regulation12,32. Taken together, it suggested that various signaling components were involved in the inflammatory response by S. aureus stimulus, they cooperated each other and assessed a distinct spatio-temporal regulation. MKP-1 acted as an important negative regulator for the signaling cascades, strictly controlled the duration and intensity of the inflammatory response to S. aureus.
It has been reported that Mkp-1 is a target gene regulated by CREB regulon33. MKP-1 could be regulated by PKA-CREB pathway18,26,27. We then assessed the induction of MKP-1 and PKA-CREB pathway molecules by S. aureus infection. In response to S. aureus, MKP-1 and PKA-cα expression was greatly up-regulated. In addition, S. aureus stimulation induced a transient activation of CREB (Ser133). Furthermore, PKA inhibitor, KT-5720 blocked the activation of CREB (Ser133) and production of MKP-1 at the same time by S. aureus stimulus in Raw264.7 cells. All these results suggested that increase of MKP-1 expression to S. aureus, at least in part, by a mechanism dependent on PKA-CREB pathway.
Rolipram, a classic PDE4-specific inhibitor which often used to treat for asthma, chronic obstructive lung disease15. A number of mechanisms have been proposed for the anti-inflammatory actions of rolipram, including repression of inflammatory cytokines releases16. Meanwhile, some studies suggested MKP-1 was involved in the anti-inflammatory effects by rolipram17,18. However, the molecular mechanisms underlying the role of MKP-1 in anti-inflammatory effects by rolipram remain unclear. Since rolipram treated always results in an elevation of intracellular cAMP levels in the cells14,15,16. Additionally, it has been shown that the primary mediator of the cellular response to cAMP is the PKA22,24. We first testified that rolipram could substantially inhibit the production of TNFα by S. aureus stimulus. In addition, rolipram could increased the intracellular cAMP levels in Raw264.7 cells. Meanwhile, PKA-cα was greatly induced by rolipram treated. These data indicated that rolipram could suppress S. aureus induced immune response by cAMP-PKA pathway. To further evaluate the contribution of MKP-1 in rolipram mediated anti-inflammatory effect, we assessed the MKP-1 expression after treating Raw264.7 cells with S. aureus or combined with rolipram. Our finding suggested that MKP-1 was involved in rolipram associated anti-inflammatory effect via a cAMP-PKA dependent pathway by S. aureus stimulus. This notion was supported by the data as follows. First, induction of MKP-1 was increased obviously at both transcription and translation levels by administration of rolipram alone in the Raw264.7 cells. Second, MKP-1 expression was increased by S. aureus stimulus and it was further enhanced in the presence of rolipram and other cAMP-elevating agents (forskolin and 8-Bromo-cAMP) in Raw264.7 cells. Additionally, rolipram could inhibit S. aureus induced phosphorylations of p38 and IκBα. Furthermore, our previous data demonstrated that MKP-1 could be an endogenous inhibitor of both p38 and IκBα molecules by S. aureus stimulus in Raw264.7 cells. Taken together, these observations suggested that the anti-inflammatory effect of rolipram on S. aureus induced inflammation could be mediated by MKP-1 via a cAMP-PKA dependent pathway.
Another interesting finding in our study was PKA-cα could directly associate with IκBα by S. aureus stimulus. In the current study, S. aureus treatment induced a high level expression of PKA-cα and activation of IκBα. Additionally, a block of PKA activity by KT 5720 remarkably enhanced the activation of IκBα induced by S. aureus. Furthermore, pretreated with rolipram and cAMP-elevating agents were all capable of inhibiting S. aureus induced IκBα activation in Raw264.7 cells. These data indicated that there was the crosstalk between IκBα and cAMP-PKA pathways during S. aureus stimulation. A direct link between IκBα and PKA pathway was established through the finding that PKA-cα could directly associate with IκBα in response to S. aureus. In our studies, PKA-cα was immunoprecipitated with IκBα in the resting Raw264.7 cells. When stimulated with S. aureus, this interaction was significantly reduced, disrupted the IκBα/PKA-cα complex. These results were consistent with the former studies that PKA-c resides constitutively tethered to IκB/NFκB complexes in the cytoplasm until IκBα was phosphorylated and degraded30,31,34. It should be emphasized that IκBα/PKA-cα complex was re-constituted after S. aureus stimulated for the later time. The three-dimensional structure of PKA revealed that this protein has a bilobate structure. The smaller N-terminal lobe is primarily used for binding to ATP, while substrate binding is carried out with sequences from the larger C-terminal lobe35. It has been testified that N-terminal ATP-binding domain of PKA binds to IκB, while the C-terminal region establishes interaction with the substrate p65 protein. Dual sites of interaction might thus allow PKA bind stably to the IκB/NFκB complex30. In our study, the results of pull-down experiments indicated that IκBα may re-constituted with N-terminal ATP-binding domain of PKA-cα and decreased of the PKA enzymatic activity after stimulated with S. aureus. These results presented a novel mechanism of PKA and IκB pathway in an auto-regulatory feedback manner. However, there still has a question: how about the C-terminal substrate binding domain? We have tried to use the antibody against PKA-cα to pull-down the P65. Unfortunately, we could not detect apparent band against the P65 (data not shown). We believe that the interaction between PKA-c and IκB might include other regulatory proteins. Identifying other molecules anchored for PKA-c will be an important area of investigation in the future.
Taken together, although we assumed that PKA was required for the induction of MKP-1 by S. aureus, we could not discard the involvement of other signaling molecules that have not analyzed in this report. In addition, some studies have implicated that CREB also can act as a p38 and ERK1/2 regulated transcription factor required for macrophage survival during LPS stimulation18,36. In fact, we did not get a completely blocking expression of MKP-1 in Raw264.7 cells when treated with the specific inhibitor against PKA at 30 minutes by S. aureus. Besides, blocking the activity of PKA was not significantly attenuation the phosphorylation of CREB (Ser133) at 30 minutes by S. aureus. These observations suggested that PKA was required for this process but some other mechanisms may participate in the induction of MKP-1 by S. aureus. We are currently analyzing the involvement of other pathways that regulate MKP-1 expression.
In summary, our present study demonstrated that MKP-1 was an important endogenous inhibitor of ERK1/2, p38 and IκBα moleculess in response to the Gram-positive bacterium, S. aureus. In addition, MKP-1 expression was enhanced by the anti-inflammatory drug, rolipram via cAMP-PKA pathway with S. aureus stimulation. Furthermore, IκBα could be regulated and directly associate with PKA-cα and this interaction may involved in the anti-inflammatory effect of rolipram by S. aureus stimulus (Fig. 8). These findings were of particular therapeutic importance because negative regulators has long been thought as therapeutic strategy during pathogen infection. Therefore, investigation the molecular mechanisms by which MKP-1 was up-regulated may not only bring novel insight into the immune homeostasis but also lead to identification of novel anti-inflammatory drug for controlling inflammation in response to S. aureus.
Materials and Methods
Reagents
The following agents U 0126, SB 203580, Bay 11-7082, KT 5720 (Calbiochem Corp, USA) and rolipram, foskolin (Sigma-Aldrich, USA) were dissolved in DMSO. Final concentration of DMSO was less than 0.1%. 8-Bromo-cAMP (8-Bromoadenosine 3′,5′-cyclic monophosphate sodium salt) was purchased from Sigma-Aldrich. MKP-1, total-IkBα, total-ERK2 antibodies obtained from Santa Cruz Biotechnology. MKP-3, MKP-5, phospho-IkBα (Ser32/36), phospho-p38 (Thr180/Tyr182), total-p38, phospho-ERK1/2 (Thr202/Tyr204), phospho-CREB (Ser133) and PKA-cα were purchased from Cell Signaling Technology. β-actin was purchased from Sigma-Aldrich. Dulbecco’s modified Eagle’s medium (DMEM), penicillin and streptomycin were from Invitrogen Life Technologies. All other reagents were purchased from Sigma-Aldrich, unless indicated otherwise.
S. aureus preparation and infection procedure
S. aureus was grown overnight at 37 °C in a shaking incubator (200 rpm) in tryptic soy broth (TSB) (Sigma). Bacteria was harvested by centrifugation at 800 × g for 10 minutes, washed twice with sterile PBS (Invitrogen, USA) and resuspended in PBS. The optical density (OD) was determined for S. aureus number estimation (A600: 0.8 OD unit ≈ 2 × 108 cfu/ml). S. aureus was added into DMEM without penicillin and streptomycin to get a final concentration 2 × 107 cfu/ml. The Raw264.7 cells monolayers (80~90% confluency) were washed and changed to no penicillin/streptomycin medium containing 2 × 107 cfu/ml S. aureus. The cells were harvested at different time points after infection.
Cell culture and drugs
Raw264.7 cells were cultured in DMEM supplemented with 10% fetal calf serum, 100 U/ml of penicillin G and 100 μg/ml streptomycin in a humidified 37 °C incubator. Prior to experimentation, cells were seeded on six well plate (2 × 105 per well) and incubated overnight, then changed to fresh no penicillin/streptomycin medium for prescribed periods of time in the presence or absence of pharmacological inhibitors or reagents.
siRNA (small interfering RNA)-mediated gene silencing of Mkp-1
Raw264.7 cells stably harboring the previously described Mkp-1 siRNA were provided by Dr. Yusen Liu37. Briefly, Raw264.7 cells were transfected with Mkp-1 siRNA expression plasmid, using FuGENE® 6 Transfection Reagent (Roche Diagnostics, USA) according to the manufacturer’s recommendations. After transfection, cells were selected in medium containing 500 μg/ml of G418 (Roche Diagnostics, USA) for two weeks, and isolated the individual G418-resistant clones. These clones were screened for MKP-1 expression with western-blot analyses by using antibody against MKP-1. Stable Raw264.7 clones expression Mkp-1 siRNA were maintained in medium containing 100 μg/ml of G418.
RNA isolation, cDNA synthesis and SYBR Green Real Time PCR
Total RNA was isolated using TRIzol® reagent (Invitrogen, USA) according to the manufacturer’s protocols, and reverse transcription reaction was prepared using 1 μg of RNA to abtain cDNA (Qiagen, USA). The resultant cDNA was diluted 1:3 in RNAse-free water. Realtime quantitative PCR (q-PCR) was performed using ABI 7700 Sequence Detection System (Applied Biosystems, USA) as described38,39,40. The sequence of primers used were as follows: mouse Mkp-1: sense, 5′-ACC ATC TGC CTT GCT TAC CTT-3′; antisence: 5′-AGC ACC TGG GAC TCA AAC TG-3′; mouse TNFα: sense, 5′-GAC CCT CAC ACT CAG ATC ATC TTC-3′; antisence: 5′-CAC GTA GTC GGG GCA GCC TTG-3′; mouse β-actin: sense, 5′-GAA GAG CTA TGA GCT GCC TGA-3′; antisence: 5′-CAG CAC TGT GTT GGC ATA GAG-3′.
Protein preparation, Immunoprecipitation and Western blotting
Whole cell lysates were prepared using cell lysis buffer containing 10 mM HEPES (pH 7.4), 50 mM β-glycerophosphote, 1% Triton X-100, 1% NP-40, 10% glycerol, 2 mM EDTA, 2 mM EGTA, 1 mM DTT, 10 mM NaF, 1 mM Na3VO4 and complete protease inhibitor cocktail (Roche Diagnostics). The lysates were centrifuged and the supernatants were boiled in SDS loading buffer. For immunoprecipitation, cell lysates were incubated with appropriate amount of antibody overnight and then precipitated following absorption onto protein A-agarose. Precipitates were washed three times, separated by SDS-PAGE, then transferred onto nitrocellulose membrane which was then incubated in TBST buffer (150 mM NaCl, 20 mM Tris-HCl, and 0.02% Tween 20, pH 7.6) containing 5% non-fat milk. Western blot analysis was conducted by using ECL reagent (Pierce, USA). The membranes probed with phospho-IkBα, phospho-p38 and phospho-ERK1/2 were stripped and re-probed with antibodies against total IkBα or total p38 or total ERK2 to confirm equal sample loading.
Statistical analysis
One-way ANONA was used to assess significant differences among treatment groups. For each significant effect of treatment, SPSS statistical software program was used for comparisons of multiple group means. A value of p < 0.05 was considered significant.
References
Elston, D. M. Community-acquired methicillin-resistant Staphylococcus aureus. Journal of the American Academy of Dermatology 56, 1–16; quiz 17–20, doi:10.1016/j.jaad.2006.04.018 (2007).
Vinh, D. C. & Embil, J. M. Rapidly progressive soft tissue infections. The Lancet. Infectious diseases 5, 501–513, doi:10.1016/S1473-3099(05)70191-2 (2005).
Opal, S. M. & Cohen, J. Clinical gram-positive sepsis: does it fundamentally differ from gram-negative bacterial sepsis? Critical care medicine 27, 1608–1616 (1999).
Takeuchi, O. et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11, 443–451 (1999).
Wang, J. E. et al. Peptidoglycan and lipoteichoic acid from Staphylococcus aureus induce tumor necrosis factor alpha, interleukin 6 (IL-6), and IL-10 production in both T cells and monocytes in a human whole blood model. Infection and immunity 68, 3965–3970 (2000).
Beutler, B. TNF, immunity and inflammatory disease: lessons of the past decade. Journal of investigative medicine: the official publication of the American Federation for Clinical Research 43, 227–235 (1995).
Camps, M., Nichols, A. & Arkinstall, S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J 14, 6–16 (2000).
Kondoh, K. & Nishida, E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim Biophys Acta 1773, 1227–1237, doi:S0167-4889(06)00453-8 (2007).
Sun, H., Charles, C. H., Lau, L. F. & Tonks, N. K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75, 487–493, doi:0092-8674(93)90383-2 (1993).
Wang, X. et al. Knockout of Mkp-1 enhances the host inflammatory responses to gram-positive bacteria. J Immunol 178, 5312–5320, doi:178/8/5312 (2007).
Abraham, S. M. et al. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med 203, 1883–1889, doi:jem.20060336 (2006).
Wang, J., Ford, H. R. & Grishin, A. V. NF-kappaB-mediated expression of MAPK phosphatase-1 is an early step in desensitization to TLR ligands in enterocytes. Mucosal Immunol 3, 523–534, doi:mi201035 (2010).
Kim, D. S. et al. Anti-inflammatory effects of glutamine on LPS-stimulated human dental pulp cells correlate with activation of MKP-1 and attenuation of the MAPK and NF-kappaB pathways. International endodontic journal 48, 220–228, doi:10.1111/iej.12303 (2015).
Zeller, E., Stief, H. J., Pflug, B. & Sastre-y-Hernandez, M. Results of a phase II study of the antidepressant effect of rolipram. Pharmacopsychiatry 17, 188–190, doi:10.1055/s-2007-1017435 (1984).
Vignola, A. M. PDE4 inhibitors in COPD–a more selective approach to treatment. Respiratory medicine 98, 495–503 (2004).
Semmler, J., Wachtel, H. & Endres, S. The specific type IV phosphodiesterase inhibitor rolipram suppresses tumor necrosis factor-alpha production by human mononuclear cells. International journal of immunopharmacology 15, 409–413 (1993).
Korhonen, R. et al. Attenuation of TNF production and experimentally induced inflammation by PDE4 inhibitor rolipram is mediated by MAPK phosphatase-1. British journal of pharmacology 169, 1525–1536, doi:10.1111/bph.12189 (2013).
Lee, J. et al. Phosphodiesterase 4B mediates extracellular signal-regulated kinase-dependent up-regulation of mucin MUC5AC protein by Streptococcus pneumoniae by inhibiting cAMP-protein kinase A-dependent MKP-1 phosphatase pathway. The Journal of biological chemistry 287, 22799–22811, doi:10.1074/jbc.M111.337378 (2012).
Schwandner, R., Dziarski, R., Wesche, H., Rothe, M. & Kirschning, C. J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. The Journal of biological chemistry 274, 17406–17409 (1999).
Chen, B. C. et al. Peptidoglycan-induced IL-6 production in RAW 264.7 macrophages is mediated by cyclooxygenase-2, PGE2/PGE4 receptors, protein kinase A, I kappa B kinase, and NF-kappa B. Journal of immunology 177, 681–693 (2006).
Chang, Y. C. et al. Lipoteichoic acid-induced nitric oxide synthase expression in RAW 264.7 macrophages is mediated by cyclooxygenase-2, prostaglandin E2, protein kinase A, p38 MAPK, and nuclear factor-kappaB pathways. Cellular signalling 18, 1235–1243, doi:10.1016/j.cellsig.2005.10.005 (2006).
Wall, E. A. et al. Suppression of LPS-induced TNF-alpha production in macrophages by cAMP is mediated by PKA-AKAP95-p105. Science signaling 2, ra28, doi:10.1126/scisignal.2000202 (2009).
Ollivier, V., Parry, G. C., Cobb, R. R., de Prost, D. & Mackman, N. Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells. The Journal of biological chemistry 271, 20828–20835 (1996).
Aronoff, D. M., Canetti, C., Serezani, C. H., Luo, M. & Peters-Golden, M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. Journal of immunology 174, 595–599 (2005).
Bryn, T. et al. The cyclic AMP-Epac1-Rap1 pathway is dissociated from regulation of effector functions in monocytes but acquires immunoregulatory function in mature macrophages. Journal of immunology 176, 7361–7370 (2006).
Lu, T. C. et al. Retinoic acid utilizes CREB and USF1 in a transcriptional feed-forward loop in order to stimulate MKP1 expression in human immunodeficiency virus-infected podocytes. Molecular and cellular biology 28, 5785–5794, doi:10.1128/MCB.00245-08 (2008).
Brion, L. et al. MAPK phosphatase-1 (MKP-1) expression is up-regulated by hCG/cAMP and modulates steroidogenesis in MA-10 Leydig cells. Endocrinology 152, 2665–2677, doi:10.1210/en.2011-0021 (2011).
Pouyssegur, J. & Lenormand, P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. European journal of biochemistry/FEBS 270, 3291–3299 (2003).
Hutter, D., Chen, P., Barnes, J. & Liu, Y. Catalytic activation of mitogen-activated protein (MAP) kinase phosphatase-1 by binding to p38 MAP kinase: critical role of the p38 C-terminal domain in its negative regulation. The Biochemical journal 352(Pt 1), 155–163 (2000).
Zhong, H., SuYang, H., Erdjument-Bromage, H., Tempst, P. & Ghosh, S. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89, 413–424 (1997).
Gambaryan, S. et al. Thrombin and collagen induce a feedback inhibitory signaling pathway in platelets involving dissociation of the catalytic subunit of protein kinase A from an NFkappaB-IkappaB complex. The Journal of biological chemistry 285, 18352–18363, doi:10.1074/jbc.M109.077602 (2010).
Keyse, S. M. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Current opinion in cell biology 12, 186–192 (2000).
Impey, S. et al. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell 119, 1041–1054, doi:10.1016/j.cell.2004.10.032 (2004).
Ghosh, M. et al. The interplay between cyclic AMP, MAPK, and NF-kappaB pathways in response to proinflammatory signals in microglia. BioMed research international 2015, 308461, doi:10.1155/2015/308461 (2015).
Knighton, D. R. et al. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 253, 407–414 (1991).
Park, J. M. et al. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis–CREB and NF-kappaB as key regulators. Immunity 23, 319–329, doi:10.1016/j.immuni.2005.08.010 (2005).
Shepherd, E. G. et al. The function of mitogen-activated protein kinase phosphatase-1 in peptidoglycan-stimulated macrophages. J Biol Chem 279, 54023–54031, doi:M408444200 (2004).
Pan, Z. K., Chen, L. Y., Cochrane, C. G. & Zuraw, B. L. fMet-Leu-Phe stimulates proinflammatory cytokine gene expression in human peripheral blood monocytes: the role of phosphatidylinositol 3-kinase. Journal of immunology 164, 404–411 (2000).
Jono, H. et al. NF-kappaB is essential for induction of CYLD, the negative regulator of NF-kappaB: evidence for a novel inducible autoregulatory feedback pathway. The Journal of biological chemistry 279, 36171–36174, doi:10.1074/jbc.M406638200 (2004).
Pan, W. W. et al. Synergistic activation of nuclear factor kappaB (NF-kappaB)by bacterial chemoattractant and tumor necrosis factor alpha (TNFalpha) is mediated by p38MAPK-dependent RelA acetylation. J Biol Chem, doi:M110.109165 (2010).
Acknowledgements
Raw264.7 cells stable expressing Mkp-1 siRNA and empty vector was a kind gift from Dr. Yusen Liu (Ohio State University, Columbus, USA). This study was supported by USPHS Grant AI43524 (ZKP) and the Guangdong Innovative Research Team Program (No. 201001Y0104675344). This work was partly supported by grant (YQP, No. 201640179) from Shanghai Municipal Commission of Population and Planned Parenthood and grant (CX, No. 81471497) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Contributions
Zhixing K. Pan initiated the project, conceived and designed the experiments; Yiqing Pan carried out most experiments, analyzed the data and wrote the manuscript; Zhixing K. Pan and Chen Xu evaluated the experiments and refined the final manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pan, Y., Xu, C. & Pan, Z.K. MKP-1 negative regulates Staphylococcus aureus induced inflammatory responses in Raw264.7 cells: roles of PKA-MKP-1 pathway and enhanced by rolipram. Sci Rep 7, 12366 (2017). https://doi.org/10.1038/s41598-017-10187-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10187-3
This article is cited by
-
Protective effects of rolipram on endotoxic cardiac dysfunction via inhibition of the inflammatory response in cardiac fibroblasts
BMC Cardiovascular Disorders (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.