Abstract
Radial endobronchial ultrasound (R-EBUS) is one important diagnostic approach in non-small cell lung cancers (NSCLC). However, the small samples obtained from R-EBUS-guided transbronchial biopsies are sometimes insufficient for pathological and molecular diagnosis. Herein, we investigated the suitability of R-EBUS-guided bronchial brushing specimens for NSCLC diagnosis and EGFR genotyping. We enrolled 941 consecutive patients with peripheral pulmonary lesions who underwent R-EBUS. Cytology-positive brushing specimens from non-squamous NSCLC patients were tested for EGFR mutations. Non-squamous NSCLC was diagnosed in 624 patients (66.3%). Positive cytology was documented in the brushing samples of 376 patients (60.3%). Higher diagnostic yields were obtained in patients exhibiting bronchus signs on chest tomography, and those with R-EBUS probe located within the lesion. EGFR genotyping was successfully performed in 363 samples (96.5% of cytology-positive brushing samples). EGFR genotyping concordance between brushing specimens and matched tissue samples was 88.7% (kappa = 0.745, P < 0.001). Furthermore, 144 non-squamous NSCLC patients (23.1%) with failed pathological diagnosis or EGER sequencing by R-EBUS-guided transbronchial biopsy required repeat biopsies. However, it was achieved successfully from the brushing specimens of 57 patients (39.6%). In conclusion, for patients with peripheral lung cancer, R-EBUS-guided bronchial brushing could provide an additional sampling method for diagnosis and EGFR genotyping.
Similar content being viewed by others
Introduction
The discovery of mutations in the epidermal growth factor receptor (EGFR), as well as that of EGFR-tyrosine kinase inhibitors (TKIs), introduced a new era of precision medicine in lung cancer treatment1,2. Clinical studies demonstrated the significant treatment efficacy of targeted therapy in patients with sensitizing EGFR mutations3,4,5. As a result, current guidelines for the diagnosis and treatment of patients with advanced non-small cell lung cancer (NSCLC) recommend that EGFR genotyping be performed in tumors with non-squamous histologies6,7.
In many patients with NSCLC, especially those with tumors of non-squamous histologies, tumors often present with peripheral pulmonary lesions (PPLs) that impede access to the target tumor by conventional bronchoscopy. Indeed, these lesions present a frequently encountered challenge to pulmonologists. With the development of radial endobronchial ultrasound (R-EBUS), which dramatically improved the visualization and localization of PPLs, the diagnostic yield of transbronchial biopsy has improved8,9. Adequate biopsy tissue samples are required for the diagnosis, subtyping, and genotyping of NSCLC samples. However, specimens obtained via transbronchial biopsy are often small and contain a limited number of tumor cells, precluding further molecular testing10,11,12. In such cases, patients require repeat procedures, such as computed tomography (CT)-guided transthoracic needle biopsy or surgical resection, to obtain additional tissue samples for molecular testing. Hence, patients undergoing repeat invasive procedures are exposed to additional risks, and treatment may be delayed as a result.
Our previous pilot study showed that RNA-based sequencing of waste bronchial brushing specimens may be a feasible method for multi-gene analysis13. However, no cohort studies have been performed to investigate the role of R-EBUS-guided bronchial brushing in cytopathological diagnosis and EGFR analysis. Therefore, in the present study, we investigated the performance of R-EBUS-guided bronchial brushing in both the cytopathological diagnosis of and EGFR mutation detection in peripheral non-squamous NSCLC.
Methods
Study design and settings
This study was conducted at National Taiwan University Hospital, a tertiary referral center. Consecutive patients with PPLs who were referred for R-EBUS between September 2010 and December 2015 were enrolled (n = 941). A PPL was defined as a lesion surrounded by lung parenchyma with no endobronchial abnormalities detected by conventional bronchoscopy. Computed tomography-based findings, including tumor location, size, and presence of a bronchus sign (i.e., a bronchus leading directly to a PPL) were documented. The treatment responses of patients with advanced NSCLC who were administered first-line EGFR-TKIs (erlotinib, gefitinib, or afatinib) were recorded based on the Response Evaluation Criteria in Solid Tumors, version 1.114. The cutoff date for data collection was November 31, 2016. This study was approved by the Institutional Review Board of National Taiwan University Hospital. Written informed consent was obtained from all patients before undergoing bronchoscopic procedures. All methods were performed in accordance with the relevant guidelines and regulations.
EBUS-guided procedures
Conventional bronchoscopy (BF-P260F or BF-P290; Olympus, Tokyo, Japan) was initially performed to examine the trachea and bronchi. R-EBUS was then performed using an endoscopic ultrasound center (EU-M30S; Olympus) and a 20-MHz radial ultrasonic probe (UM-S20-20R; Olympus). The R-EBUS probe position was recorded as within or adjacent to the target tumor. After a lesion was located, the radial probe was withdrawn from the working channel of the bronchoscope, and the R-EBUS procedure consisting of transbronchial biopsy, bronchial brushing, and bronchial washing was then performed.
Specimen preparation
Each specimen obtained by bronchial brushing was first smeared onto slides. Air-dried smears and those fixed in 95% ethyl alcohol were prepared for routine evaluation. Next, the brushing head was removed and dipped into TRI reagent solution (Molecular Research Center, Cincinnati, OH), and specimens were stored as described previously15. The bronchial brushing cytology slides were examined by a board-certified cytopathologist. EGFR mutation analysis was then performed on tumor cell-containing bronchial brushing specimens obtained from patients diagnosed with non-squamous NSCLC.
EGFR mutation analysis of brushing specimens
Reverse transcription-polymerase chain reaction (RT-PCR) on RNA extracted from the brushing specimens was performed with the Qiagen One-Step RT-PCR Kit (Qiagen, Hilden, Germany) using previously reported conditions and primers13,16. The RT-PCR amplicons were purified and sequenced with a BigDye Terminator Sequencing Kit (Applied Biosystems, Foster City, CA). Sequencing products underwent electrophoresis on an automated ABI PRISM 3700 genetic analyzer (Applied Biosystems). Both the forward and reverse sequences obtained were analyzed, and chromatograms were examined manually.
EGFR mutation analysis from matched histological specimens
Matched histological specimens, including biopsy specimens and surgical tissues, were used for EGFR mutation detection. EGFR mutations were analyzed using standard methods at our institution, including either direct sequencing or matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS), as previously described13,17,18,19.
Statistical analysis
For univariate analysis, categorical variables were analyzed using the chi-squared test or Fisher’s exact test as appropriate. For multivariate analysis, variables with P-values of <0.10 were incorporated into the multivariate logistic regression model to identify independent factors. Intra-individual agreements between the different methods used to detect EGFR mutations were determined by calculating Cohen’s kappa coefficient. Progression-free survivals after treatment with EGFR-TKIs were analyzed using the Kaplan-Meier method. All analyses were conducted using SPSS for Windows, version 15.0 (SPSS Inc., Chicago, IL, US). A two-tailed P-value of <0.05 was considered statistically significant.
Results
Patient characteristics
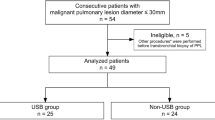
A total of 941 consecutive patients who underwent R-EBUS were enrolled in this study. The final diagnoses of these patients, established either by R-EBUS or other diagnostic procedures, are shown in Fig. 1. Among these patients, 722 were diagnosed with lung cancer, 624 of whom (86.4%) had non-squamous NSCLC; the clinical features of the latter group of patients are shown in Table 1. R-EBUS-guided transbronchial biopsy and bronchial brushing cytology established the cytopathological diagnosis of 427 (68.4%) and 376 (60.3%) non-squamous NSCLC patients, respectively. Non-squamous NSCLC was identified in 489 patients (78.4%) when the results of both transbronchial biopsy and bronchial brushing cytology were considered together, while 62 patients (9.9%) were diagnosed solely on the basis of bronchial brushing cytology. Samples from the 376 patients with tumor cells found on their brushing smears underwent RT-PCR and Sanger sequencing for EGFR mutation analysis.
Predictive factors for positive brushing cytology results
Factors associated with the diagnostic yield of non-squamous NSCLC samples are shown in Table 2. In univariate analysis, the diagnostic yield of brushing samples was significantly associated with tumor size and presence of a bronchus sign on chest CT, and R-EBUS probe position. Multivariate analysis showed that significantly higher diagnostic yields were associated with a bronchus sign and the localization of the R-EBUS probe located within the lesion (P < 0.01, odds ratio [OR]: 1.97, confidence interval [CI]: 1.39–2.80; P < 0.01, OR: 2.37, 95% CI: 1.56–3.60, respectively).
EGFR mutation analysis of samples obtained by R-EBUS brushing
Successful EGFR genotyping was achieved in bronchial brushing samples from 363 of 376 patients (96.5%), while samples from 13 patients (3.5%) failed EGFR amplification and sequencing because of insufficient material. EGFR mutations were detected in 216 patient samples; among them, 99 (38.5%) were exon 19 deletions, 84 (23.9%) were L858R, and 33 (9.4%) were other uncommon mutations.
Comparison among EGFR analysis methods
Of the 363 patients for whom EGFR genotyping of brushing specimens was successful, 284 had matched samples available for EGFR testing using standard testing methods. Specifically, 204 specimens were genotyped using MALDI-TOF-MS, whereas 80 were analyzed with Sanger sequencing. Comparison of EGFR testing results between the bronchial brushing and matched histological samples (Table 3) yielded a concordance rate of 88.7%, with a kappa value of 0.745 (P < 0.001).
Performance of R-EBUS-guided bronchial brushing in patients with failed transbronchial biopsies
Of the 624 patients with non-squamous NSCLC who underwent R-EBUS transbronchial biopsy, 144 (23.1%) required repeat biopsies for pathological diagnosis or EGFR testing because of inaccessible transbronchial lesions (n = 6), negative transbronchial biopsy results (n = 107), or inadequate material (n = 31). Among patients requiring repeat biopsies, the R-EBUS brushing specimens of 57 patients (39.6%) provided positive cytology and successful EGFR sequencing results (Table 4). Fifteen patients with initial failed transbronchial biopsies underwent repeat R-EBUS-guided procedures, including transbronchial biopsy and bronchial brushing. Pathological diagnosis and EGFR genotyping were successfully performed with repeat R-EBUS-guided biopsies in 8 (53.3%) patients.
Treatment efficacy of EGFR-TKIs according to EGFR mutation status of R-EBUS brushing specimens
Of the 419 patients with advanced (stage IIIB/IV) non-squamous NSCLC enrolled in this study, EGFR mutation profiles were obtained from the bronchial brushing specimens of 142 cases. Tumors from 121 patients harbored EGFR mutations, whereas 21 possessed wild-type EGFR. All patients for whom tumor EGFR mutation status was obtained were then treated with first-line EGFR-TKIs. Among these patients, those with EGFR mutations exhibited improved disease response (63.0% vs. 33.3%, P < 0.001) and disease control rates (95.0% vs. 5%, P < 0.001), as well as prolonged progression-free survival (11.7 months vs. 7.2 months, P = 0.006), compared to those without EGFR mutations.
Discussion
Our findings support a role for R-EBUS-guided bronchial brushing samples in the cytological diagnosis and EGFR mutation analysis in patients with peripheral non-squamous NSCLC, thus allowing patients to avoid more invasive procedures.
Several previous studies have demonstrated the importance of EGFR mutation analysis in predicting the treatment efficacy of EGFR-TKIs in patients with NSCLC1,3,4,5. The reliable harvesting of samples for pathological diagnosis and EGFR testing is critical for patients with advanced non-squamous NSCLC. Marked progress in diagnostic bronchoscopy has been achieved in the last decade, and R-EBUS is one of the most important of these advances20. Previous studies have demonstrated that EBUS-guided bronchial brushing improved the diagnostic yields of samples from patients with PPLs8,20,21,22. However, few studies have investigated whether R-EBUS specimens are conducive for molecular diagnostics. Guiser et al. demonstrated that the molecular diagnosis of R-EBUS specimens was feasible for approximately 80% of patients with peripheral lung cancer23. However, the majority of these specimens were biopsy, rather than bronchial brushing, specimens, and the results from EGFR mutation analyses of brushing specimens were not compared with those from patient-matched histological samples.
Previous studies have investigated the utility of cytological specimens for molecular testing, but focused on fine-needle aspiration and pleural effusion analysis rather than brushing cytology specimens24,25,26. Our previous pilot study demonstrated the potential utility of EBUS-guided brushing specimens in molecular diagnostics13. However, the factors that predict diagnostic yields of R-EBUS-guided bronchial brushing samples were not assessed because of the relatively small population size. Therefore, in the current study, we investigated the feasibility of using R-EBUS-guided brushing specimens for the cytological diagnosis and EGFR mutation analysis of tumors in patients with peripheral non-squamous NSCLC.
In the present study, the utility of R-EBUS brushing samples for molecular diagnostics was determined. We observed that the diagnostic yields of specimens from R-EBUS-guided transbronchial biopsy, brushing cytology, and both techniques combined were 68.4%, 60.3%, and 78.4%, respectively, consistent with the results of previous studies8,20,21. Moreover, we found that the presence of a bronchus sign on chest CT, as well as the localization of the R-EBUS probe within the lesion, were independent factors that improved the diagnostic yield of R-EBUS-guided bronchial brushing specimens. Previous studies have shown that the location of the tumors may influence the diagnostic yield of R-EBUS-guided transbronchial biopsies9,27,28. To our knowledge, our study is the first to investigate the factors that predict the diagnostic yield of R-EBUS-guided bronchial brushing specimens.
EGFR mutation detection was performed successfully in 96.5% of the 363 R-EBUS-guided brushing specimens that contained tumor cells. We observed good concordance between the EGFR mutation status of brushing-derived vs. patient-matched histological specimens (kappa = 0.745). Using RT-PCR, we also identified rare mutations that were not detected with standard DNA-based methods29. Moreover, 142 advanced NSCLC patients with EGFR-mutant brushing specimens exhibited better treatment responses and progression-free survival compared to patients with EGFR-wild-type specimens. The treatment efficacy of EGFR-TKIs was similar to that observed in previous studies3,4,5.
Some discrepancies between the EGFR mutational profiles of the brushing samples and matched biopsy/resection specimens were noted. First, some patients with EGFR-wild-type biopsy specimens harbored EGFR mutations in their R-EBUS brushing specimens. The sensitivity of mutation assessment may be affected by interference from non-tumor cells in the biopsy specimens. Using an RT-PCR-based system for mutation detection may overcome discrepancies caused by heterogeneous specimens15. Second, some patients with a single mutation in their biopsy specimens harbored uncommon, complex mutations in their R-EBUS brushing specimens. The RT-PCR-based method with Sanger sequencing identified these uncommon, complex mutations, which is important for predicting the treatment efficacy of EGFR-TKIs30. Third, some EGFR mutations detected in biopsy specimens were not detected in the corresponding R-EBUS brushing specimens, potentially due to the limited number of tumor cells in the latter sample type. Moreover, tumor spatial heterogeneity may also lead to discrepancies in EGFR mutation status31,32.
In this study, 144 patients required repeat invasive procedures because of negative biopsy results or insufficient samples for EGFR mutation analysis. Among them, 76 patients (52.8%) had stage IV non-squamous NSCLC with various distant metastases, including pleura, pericardium, bone, brain, liver, adrenal metastases, and so on. Because of tumor size and location, some of these metastases were more difficult and invasive to approach compared with R-EBUS. In this context, acquisition of R-EBUS-guided bronchial brushing samples and the associated increased diagnostic yields facilitated EGFR mutation analysis. The simultaneous use of both techniques enabled successful pathological diagnosis and EGFR genotyping following a single procedure, averting the need for additional invasive procedures.
Advances in the molecular diagnosis of lung cancer include “liquid biopsy”, by which genotyping is performed on circulating tumor DNA in the plasma33,34,35,36,37; however, recent studies showed variable reliability and sensitivity when detecting mutations using this method; cost and technical problems were also considerations. Cytological/histological specimens and liquid biopsy may be considered as complementary methods of molecular testing.
Our results should be interpreted in light of the limitations of our study. First, the matched specimens for comparing EGFR mutation status were not obtained exclusively from R-EBUS transbronchial biopsies. Second, the study was conducted at a single institution; multi-center studies remain under consideration. Third, testing for other mutations, such as ALK translocation, was not performed in this study and should be investigated in further work. Finally, advances in bronchoscopy techniques, including guide sheath and electromagnetic navigation bronchoscopy, can increase sample diagnostic yield without causing additional complications, such as pneumothorax38,39,40. As such, the utility of these techniques to molecular diagnostics, together with R-EBUS-guided bronchial brushing, requires further investigation.
Data availability
The datasets that were generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 304, 1497–1500 (2004).
Sordella, R., Bell, D. W., Haber, D. A. & Settleman, J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 305, 1163–1167 (2004).
Mok, T. S. et al. Gefitinib or Carboplatin–Paclitaxel in Pulmonary Adenocarcinoma. New England Journal of Medicine. 361, 947–957 (2009).
Rosell, R. et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. The lancet oncology. 13, 239–246 (2012).
Wu, Y. L. et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. The lancet oncology. 15, 213–222 (2014).
Lindeman, N. I. et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Journal of Thoracic Oncology. 8, 823–859 (2013).
Leighl, N. B. et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. Journal of Clinical Oncology. 32, 3673–3679 (2014).
Paone, G. et al. Endobronchial Ultrasound-Driven Biopsy in the Diagnosis of Peripheral Lung Lesions. Chest. 128, 3551–3557 (2005).
Steinfort, D. P., Khor, Y. H., Manser, R. L. & Irving, L. B. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. European Respiratory Journal. 37, 902–910 (2011).
Coghlin, C. L. et al. Quantitative analysis of tumor in bronchial biopsy specimens. Journal of Thoracic Oncology. 5, 448–452 (2010).
Reck, M. et al. Tissue sampling in lung cancer: a review in light of the MERIT experience. Lung cancer. 74, 1–6 (2011).
Curley, F. J., Johal, J. S., Burke, M. E. & Fraire, A. E. Transbronchial lung biopsy: can specimen quality be predicted at the time of biopsy? Chest. 113, 1037–1041 (1998).
Tsai, T. H. et al. Multi-gene analyses from waste brushing specimens for patients with peripheral lung cancer receiving EBUS-assisted bronchoscopy. Lung cancer. 82, 420–425 (2013).
Eisenhauer, E. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer. 45, 228–247 (2009).
Tsai, T. H. et al. RNA is favourable for analysing EGFR mutations in malignant pleural effusion of lung cancer. European Respiratory Journal. 39, 677–684 (2012).
Mitsudomi, T. et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non–small-cell lung cancer with postoperative recurrence. Journal of Clinical Oncology. 23, 2513–2520 (2005).
Shih, J. Y. et al. Epidermal growth factor receptor mutations in needle biopsy/aspiration samples predict response to gefitinib therapy and survival of patients with advanced nonsmall cell lung cancer. International journal of cancer. 118, 963–969 (2006).
Eberhard, D. A. et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non–small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. Journal of clinical oncology. 23, 5900–5909 (2005).
Su, K. Y. et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non–small-cell lung cancer. Journal of clinical oncology. 30, 433–440 (2012).
Herth, F., Ernst, A. & Becker, H. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. European Respiratory Journal. 20, 972–974 (2002).
Huang, C. T., Ho, C. C., Tsai, Y. J., Yu, C. J. & Yang, P. C. Factors influencing visibility and diagnostic yield of transbronchial biopsy using endobronchial ultrasound in peripheral pulmonary lesions. Respirology. 14, 859–864 (2009).
Kuo, C. H. et al. Endobronchial ultrasound-guided transbronchial biopsy and brushing: a comparative evaluation for the diagnosis of peripheral pulmonary lesions. European Journal of Cardio-Thoracic Surgery. 45, 894–898 (2013).
Guisier, F. et al. Molecular analysis of peripheral non‐squamous non‐small cell lung cancer sampled by radial EBUS. Respirology. 21, 718–726 (2016).
Rekhtman, N. et al. Suitability of thoracic cytology for new therapeutic paradigms in non-small cell lung carcinoma: high accuracy of tumor subtyping and feasibility of EGFR and KRAS molecular testing. Journal of Thoracic Oncology. 6, 451–458 (2011).
Smouse, J. H. et al. EGFR mutations are detected comparably in cytologic and surgical pathology specimens of nonsmall cell lung cancer. Cancer Cytopathology. 117, 67–72 (2009).
Cai, G. et al. Identification of EGFR mutation, KRAS mutation, and ALK gene rearrangement in cytological specimens of primary and metastatic lung adenocarcinoma. Cancer cytopathology. 121, 500–507 (2013).
Yamada, N. et al. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest Journal. 132, 603–608 (2007).
Chen, C. H. et al. Improved diagnostic yield of bronchoscopy in peripheral pulmonary lesions: combination of radial probe endobronchial ultrasound and rapid on-site evaluation. Journal of thoracic disease. 7, S418–425 (2015).
Angulo, B. et al. A comparison of EGFR mutation testing methods in lung carcinoma: direct sequencing, real-time PCR and immunohistochemistry. PloS one 7, e43842, https://doi.org/10.1371/journal.pone.0043842 (2012).
Wu, S. G. et al. Good response to gefitinib in lung adenocarcinoma of complex epidermal growth factor receptor (EGFR) mutations with the classical mutation pattern. The oncologist. 13, 1276–1284 (2008).
de Bruin, E. C. et al. Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science. 346, 251–256 (2014).
Marusyk, A., Almendro, V. & Polyak, K. Intra-tumour heterogeneity: a looking glass for cancer? Nature Reviews Cancer. 12, 323–334 (2012).
Punnoose, E. A. et al. Evaluation of circulating tumor cells and circulating tumor DNA in non–small cell lung cancer: Association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clinical Cancer Research. 18, 2391–2401 (2012).
Douillard, J. Y. et al. Gefitinib treatment in EGFR mutated caucasian NSCLC: circulating-free tumor DNA as a surrogate for determination of EGFR status. Journal of Thoracic Oncology. 9, 1345–1353 (2014).
Qiu, M. et al. Circulating tumor DNA is effective for the detection of EGFR mutation in non–small cell lung cancer: a meta-analysis. Cancer Epidemiology and Prevention. Biomarkers. 24, 206–212 (2014).
Thress, K. S. et al. EGFR mutation detection in ctDNA from NSCLC patient plasma: A cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer. 90, 509–515 (2015).
Jenkins, S. et al. Plasma ctDNA analysis for detection of the EGFR T790M mutation in patients with advanced non-small cell lung cancer. Journal of Thoracic Oncology. 12, 1061–1070 (2017).
Kikuchi, E. et al. Endobronchial ultrasonography with guide-sheath for peripheral pulmonary lesions. European Respiratory Journal. 24, 533–537 (2004).
Asano, F. et al. Diagnosis of peripheral pulmonary lesions using a bronchoscope insertion guidance system combined with endobronchial ultrasonography with a guide sheath. Lung Cancer. 60, 366–373 (2008).
Eberhardt, R., Anantham, D., Ernst, A., Feller-Kopman, D. & Herth, F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. American journal of respiratory and critical care medicine. 176, 36–41 (2007).
Acknowledgements
The authors thank all the physicians and nursing staff for excellent patient care during the study period. They are affiliated with the Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan.
Author information
Authors and Affiliations
Contributions
Kai-Lun Yu, Tzu-Hsiu Tsai and Jin-Yuan Shih participated in the study concept and design, subject recruitment, data analysis, and manuscript composition. Chao-Chi Ho, Wei-Yu Liao, Ching-Kai Lin and Chia-Lin Hsu participated in the subject recruitment manuscript composition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, KL., Tsai, TH., Ho, CC. et al. The value of radial endobronchial ultrasound-guided bronchial brushing in peripheral non-squamous non-small cell lung cancer. Sci Rep 8, 5837 (2018). https://doi.org/10.1038/s41598-018-24300-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24300-7
This article is cited by
-
Combination of electromagnetic navigation bronchoscopy-guided biopsy with a novel staining for peripheral pulmonary lesions
World Journal of Surgical Oncology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.