Abstract
Acute aortic syndromes (AAS) have been related to significant circadian and seasonal conditions. We used time series analyses to study the impact of meteorological variations on AAS occurrence. We retrospectively assessed 140 patients presenting with AAS over a 6-year period in a French university hospital. Average daily temperature (T) and atmospheric pressure (AP) at the location of the event were collected within the previous 10 days, and their association with AAS investigated with generalized additive models. A decrease in temperature of more than 5 °C within the previous seven days was significantly associated with an increased risk of AAS occurrence (OR equal to 1.86 [1.06; 3.44]). Subgroup analysis revealed that the risk was only significant among normotensive individuals (n = 41) free from blood pressure lowering medication (OR equal to 2.3 [1.05; 5.37]), but not among hypertensive individuals under blood pressure lowering medication despite a larger patient number (n = 99). Similarly, only among the subgroup of normotensive individuals a decrease of AP between 2 and 4 kPa within the previous 3 days was associated with an increased risk of AAS (OR equal to 2.93 [1.1; 8.15]) and an increased between 2 and 4 kPa was associated with a decreased risk (OR equal to 0.59 [0.36; 1.00]). Variations of meteorological conditions (temperature and AP) within the previous week seem to have effects on triggering AAS especially among the population free from blood pressure lowering medication.
Similar content being viewed by others
Introduction
Similar to other cardiovascular adverse events, acute aortic syndromes (AAS) exhibit significant circadian and seasonal variations. Characterizing and understanding these variations is essential to ensure an optimal management of medical resources throughout the year and treatment strategy during vulnerable periods. Several studies have investigated the influence of climatic conditions on AAS1,2,3,4,5,6, reporting a significant occurrence of AAS in the morning and a correlation with low temperature and winter season2,3,4,5. However, most of these studies are descriptive or investigate the impact of daily temperature only5, even if climatic variation throughout several consecutive days may have also been of major importance3.
Time series analyses are widely used to model the influence of climatic factors on health outcomes7,8. These models allow for the modeling of the impact of delayed effects of climatic factor variations, while accounting for temporal processes and adjusting for potential confounding factors. The impact of variations in climatic conditions may depend on local context such as the initial value at which the variation starts. A decrease in temperature starting from a high temperature could indeed have a different effect compared to a decrease from a low value. Therefore, rather than absolute climatic conditions, their relative changes may be of interest9. To our knowledge, these models have not been used in acute aortic syndromes.
The objective of this study was to investigate the effects of both relative and absolute change in temperature and atmospheric pressure on the risk of AAS. Specifically, we assessed the possible delayed effect of these climatic factors within 10 days prior to the event; the effect of the initial values of climatic conditions; and the patterns of relationships between climatic conditions and the risk of AAS.
Methods
This study was a retrospective, monocentre observational trial in a university hospital located in Nantes (France), and has been approved by the local research ethics committee the GNEDS (Groupe Nantais d’Ethique dans le Domaine de la Santé) and the CNIL (Commission Nationale de l’Informatique et des Libertés); the study reference number was RC17_0181. Participation required informed consent obtain by phone and compliance with the study inclusion and exclusion criteria. We combined hospital records-based information with local weather forecast archives. All methods were performed in accordance with the relevant guidelines and regulations.
Population
All consecutive patients admitted for AAS from January 2007 to December 2016 were considered for inclusion in the study. AAS included Stanford type A acute aortic dissections (AAD) or intramural hematoma and Stanford type B AAD or intramural hematoma. The diagnosis of AAS was based on computed tomography angiography. Patients living beyond a 100 km radius from a meteorological station were excluded to avoid information bias due to inaccurate data.
Type A AAD underwent surgery in all cases with subsequent histopathological examination of the aortic wall; type B AAD underwent either best medical treatment or endovascular repair if complicated. Case collected characteristics were: type of AAS, age, gender, body mass index, cardiovascular risk factors, date and location at the time of AAS occurrence. Data were collected from the hospital registry, and included every patient who arrived alive at the hospital (patients who succumbed to the AAS event before reaching the hospital were not included in this study).
Climatic data
Nantes is located in the west part of France and has a temperate oceanic climate. It presents with an urban, suburban and country catchments area of about 2 900 000 inhabitants. Climatic data were obtained from Météo-France web service, the national meteorological service of France, which compiles raw data from Nantes meteorological station various sources and re-analyses the data using weather prediction models. Data consisted of temperature and atmospheric pressure collected daily every 3 hours over the past 10 years. Average daily temperatures and atmospheric pressures were calculated for each area and then combined with clinical data. This approach assumed that in a specified geographic area all persons experience the same exposure.
Statistical analyses
The associations between climatic factors and the risk of AAS were investigated with generalized additive models. Due to the sparse distribution of cases over days (a very low number of days with more than one case), daily occurrence of AAS was considered as a binary outcome. Long-term trends and seasonality were filtered out in order to account for potential confounding factors, such as changes in health status over time or atmospheric pollution, while retaining uncompounded shorter-term fluctuations possibly associated with short-term variations of climatic factors.
Prior to the study of short-term climatic factors per se, a regional model was set to remove long-term trend and seasonality. Six degrees of freedom (6 years of inclusion) were chosen for the annual long-term trends in the areas of Nantes to capture bi-monthly variations of occurrence of AAS. The analyses required two stages. The first stage consisted in the identification of a possible delayed effect of the variations of climatic factors on the risk of AAS (identification of time lags). Day 0 was the day of occurrence of the AAS, and day -n was the day “n” days prior to the AAS. The description of the statistical modeling strategy is detailed in the supplemental material. Briefly, automatic term selections were used to identify the time lags for temperature and atmospheric pressure associated with variations of risk of AAS. Four degrees of freedom were used for each climatic variable to account for non-linearity.
In the second step, the effect of variations in temperature and atmospheric pressure on the risk of AAS - according to the initial value at which the variation starts (absolute vs. relative effect) - was investigated. Therefore, for each time lag of temperature and atmospheric pressure identified in the first step, two possible variables were considered: the variation between day 0 and day -n alone or its interaction with the initial value at day -n. Then, for each time lag considered, the different models were compared using Akaike’s Information Criteria (AIC). All models are presented in the Supplementary Materials section. Finally, subgroup analyses we performed according to the hypertensive status and the type of AAS. We defined as “normotensive patient” every patient without previous medical history of hypertension and free from blood pressure lowering medication; and as “hypertensive patient” every patient under blood pressure lowering medication at the time of the event. P-values < 0.05 were considered to be statistically significant. Statistical analyses were performed using R software (version 3.5.1).
Results
A total of 140 patients living within the studied area have been admitted with AAS diagnosis over the 6-year studied period. Eleven patients were excluded because living beyond a 100 km radius from the meteorological station. The cumulative daily incidence of AAS (number of days with at least one case / total number of days during the study period) was 0.038 with 138 days with one or more patients admitted for AAS. The number of AAS in both areas showed variations within months and years (Fig. 1), with significantly more cases in May (P = 0.03) and December (P = 0.03) compared to April, and in 2013 (P = 0.01) and 2014 (P = 0.04) compared to 2008. Patients’ characteristics are described in Table 1.
The monthly average of temperatures ranged from 4.1 to 18.4 °C, and the monthly average of atmospheric pressures from 100.9 to 101.3 kPa (Fig. 2). There was a significant higher temperature between march and October (all P < 0.001) compared to January, and a higher atmospheric pressure in April (P = 0.001), May (P = 0.04), November (P < 0.001) and December (P = 0.001) compared to January. The variations of climatic conditions, modeled using long-term trend and seasonality components, are presented in the Supplemental Material Section (S1).
Delayed effect of climatic conditions and impact of initial values on AAS
All the intermediate results are presented in the Supplemental Material S1 to S4.
Effect of temperature change on AAS
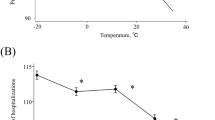
The difference in temperatures between day 0 and day −7 was associated with a significant increased risk of AAS occurrence at day 0. A decrease in temperature (more than 5 °C) within the previous seven days was associated with an increased risk of AAS (average OR equal to 1.86 [1.06; 3.44]) (Fig. 3, Table 2). Subgroup analysis revealed that the risk was only significantly increased among “normotensive patients” (average OR equal to 2.3 [1.05; 5.37]) despite a limited number of patients (n = 41) (among “hypertensive patient” OR was equal to 1.33 [0.79; 2.31]).
Variations of risk of acute aortic syndrome, expressed as Odds-Ratio (OR) with their 95% Confidence Intervals (CI), associated with (A) the difference between temperature at day 0 and day -7; and (B) the difference between atmospheric pressure at day 0 and day −3 (model 2 with Npa = 3 in Table 1 Supplemental Material).
Effect of atmospheric pressure change on AAS
An increase in atmospheric pressure (between 2 and 4 kPa within the previous 3 days) was associated with a decreased risk of AAS (average OR equal to 0.59 [0.36; 1.00]) (Fig. 3, Table 3). Similarly to temperature analysis, subgroup analysis revealed that the impact of atmospheric pressure on AAS was significant among the subgroup of “normotensive patients” but not among the subgroup of “hypertensive patients”. Among “normotensive patients”, an increase in AP was significantly associated with a reduced risk of AAS (OR equal to 0.36 [0.15; 0.91]) and a decrease in AP was significantly associated with an increased risk (OR equal to 2.93 [1.1; 8.15]), (Fig. 3, Table 3).
Discussion
This study reports a risk of AAS triggering event related to both variation in temperature and atmospheric pressure within the last week among a population of patient free from arterial blood pressure medication. Indeed, although considerable advances in identifying the conditions that may predispose to AAS have occurred, little is known about the incident event leading to the acute event. In 2002, Mehta et al. analyzed patients enrolled in the International Registry of Acute Aortic Dissection (IRAD), and they reported for the first time in large scale a high circadian variation and a frequency of AAS significantly higher during winter with a peak in January2. From this report, many studies1,2,3,4,5,6 had worked on this topic and exhibited significant circadian and seasonal monthly variations but only one reported statistically significant differences between decrease in outdoor temperature and incidence of AAS. In 2010, Benouaich et al. raised the key role of temperature decreasing during the day before AAS and suggested an interesting way that relative change in temperature could be a triggering factor rather than the absolute temperature3.
In the French area considered in this study, variations of temperature and atmospheric pressure were found to be associated to the risk of AAS within the following days. We reported that a decrease in temperature within the seven previous days was associated with an increased risk of AAS. Regarding the variations of atmospheric pressure, an increase within the previous three days was associated with reduced risk of AAS. These results suggest, as also reported for rupture of abdominal aortic aneurysms10,11 and acute myocardial infarction12, a complex relationship between seasons and pathophysiological exogenous and endogenous individual factors.
Mechanisms leading to the possible influence of external weather on AAS onset are most likely multifactorial. It has been confirmed that blood pressure variations influence the incidence of AAS, and there are also correlations between hypertension and atmospheric temperature. Bai et al. reported that compared to the temperature with minimum risk of morbidity, cold temperatures were associated with a 37% increase in hypertension-related hospitalizations whereas no significant association with hot temperatures (99th percentile) was observed13. In a large population-based analysis, Alpérovitch et al. reported that blood pressure was strongly influenced by outdoor temperature especially in the elderly, with a systolic blood pressure increase of 0.8 mm Hg in subjects aged 65 to 74 years for a 15 °C decrease in outdoor temperature14. The acute cold-induced rise in blood pressure is caused by the activation of the autonomic nervous system where the cooling-related sympathetic response results in vasoconstriction and subsequently increased blood pressure and higher cardiac workload15.
The only physiological parameter associated with atmospheric pressure is arterial blood gas concentration16, even if the exact mechanism by which reduced arterial oxygen tension may trigger an aneurysm rupture or a dissection remains unknown. A hypothesis is that changes in atmospheric pressure may increase transmural arterial stress by transiently lowering tissue pressure in respect to blood pressure creating a net expansive force on a weakened aneurysmal wall prone to rupture. Additionally, atmospheric pressure may also increase blood pressure and consequently the shear stress across the weakened aneurysm wall17.
Interestingly in our study, subgroup analysis revealed that external weather impacted only patients free from blood pressure lowering medication at the time of the event. These results suggest these medications, reducing endothelial dysfunction, may decrease the impact of external factors on the arterial wall shear stress.
In addition to the well described winter peak in AAS, a second peak in April/May was observed in our study. In 2003, McCarthy et al.10 from the university of Leicester were the first to highlight this second peak of AAS and raised the hypothesis that their finding was due to either a local geographical factor or a result of the smaller sample size (n = 223). Our study suggests that another explanation may be the high variation of temperature and atmospheric pressure during these months.
This varied impact of climatic factors suggests the existence of multiple determinants influencing the risk of AAS occurrence. Edwin et al.18 exposed that pathogenesis of AAS could be the result of the interaction of three factors: (i) a predisposition provided by an abnormality or weakening of the aortic media, (ii) any agent of intimal injury or tear resulting in the intima medial flap, and (iii) hemodynamic factors that propagate the dissection once it has been initiated. Clearly, climatic conditions only represent a precipitating factor for AAS, in addition to predisposing factors such as media histological alterations for dissections. In the subgroup analysis of patients with histopathological alteration of the media, we failed to demonstrate a significant variation in the impact of the climatic conditions as compared with other patients, possibly because of a lack of statistical power.
Strength of this study was the inclusion of cases which covered quite long time periods (6 years). Appropriate statistical models were used to investigate the effects of both temperature and atmospheric pressure while removing the effects of seasonality and long-term trends. The results involving interactions between the changes in climatic factors and their initial values confirm the interest of considering such models that allow modeling complex and non-linear relationships.
A limitation of this study was its ecological design, i.e. climatic data were not individually collected but were attributed to individuals that lived near to the meteorological station. The risk is, therefore, to attribute data to individuals that would not be representative of their close environments. In order to limit this risk, only cases living close to the meteorological stations were included (within 100 km). This selection consequently limited the number of cases, with a number of cases quite limited for time series models involving complex term interactions. Another limitation of our study was that the role of other weather variables, like relative humidity or the integrative body heat index, were not included in the analysis. Moreover, due to the retrospective design of the study, it was not possible to include in the analysis some parameters like the control of a hypertension as well as its severity and their correlation with the event, or the patient’s medication. Finally, patients who died from their condition prior to hospital admission were not included in this work.
We report here that short term changes in temperature or atmospheric pressure have a significant impact on AAS occurrence, depending on an upward or downward variation. Subgroup analysis revealed that external factors had an impact only in patients free from blood pressure lowering medication. The use of appropriate models allowed us to demonstrate these complex relationships, accounting for possible confounding factors, temporal correlation of the data, identifying lag time effect and modeling non-linear relationships. These finding may help to tailor preventive strategies for high risk patients and to anticipate a higher influx of AAS to optimize surgical and medical resources throughout the year. Future studies with larger scale are required to assess this statistical model and confirm our promising approach.
References
Krdzalic, A. et al. Atmospheric pressure changes are associated with type A acute aortic dissections and spontaneous abdominal aortic aneurysm rupture in tuzla canton. Med. Arch. 68, 156–8 (2014).
Mehta, R. H. et al. Chronobiological patterns of acute aortic dissection. Circulation 27, 1110–1115 (2002).
Benouaich, V. et al. Impact of meteorological conditions on the occurrence of acute type A aortic dissections. Interact. Cardiovasc. Thorac. Surg. 10, 403–6 (2010).
Verberkmoes, N. J. et al. Impact of temperature and atmospheric pressure on the incidence of major acute cardiovascular events. Neth. Heart J. 20, 193–6 (2012).
Law, Y., Chan, Y. C. & Cheng, S. W. Influence of meteorological factors on acute aortic events in a subtropical territory. Asian J. Surg. 40, 329–337 (2017).
Repanos, C. & Chadha, N. K. Is there a relationship between weather conditions and aortic dissection? BMC Surg. 15, 21 (2005).
Katsouyanni, K. et al. Short term effects of air pollution on health: a European approach using epidemiologic time series data: the APHEA protocol. J. Epidemiol Community Health 50, S12-18 (1996).
Kunst, A. E., Looman, C. W. & Mackenbach, J. P. Outdoor air temperature and mortality in The Netherlands: a time-series analysis. Am. J. Epidemiol. 137, 331–41 (1993).
Mehta, R. H. et al. The winter peak in the occurrence of acute aortic dissection is independent of climate. Chronobiol. Int. 22, 723–729 (2005).
Bown, M. J., McCarthy, M. J., Bell, P. R. & Sayers, R. D. Low atmospheric pressure is associated with rupture of abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 25, 68–71 (2003).
Harkin, D. W. et al. Periods of low atmospheric pressure are associated with high abdominal aortic aneurysm rupture rates in Northern Ireland. Ulst. Med. J. 74, 113–121 (2005).
Abrignani, M. G. et al. Influence of climatic variables on acute myocardial infarction hospital admissions. Int. J. Cardiol. 137, 123–129 (2009).
Bai, L. et al. Hospitalizations from Hypertensive Diseases, Diabetes, and Arrhythmia in Relation to Low and High Temperatures: Population-Based Study. Sci. Rep. 26, 30283 (2016).
Lpérovitch, A. et al. Relationship between blood pressure and outdoor temperature in a large sample of elderly individuals: the Three-City study. Arch. Intern. Med. 169, 75–80 (2009).
Hintsala, H. et al. Central aortic blood pressure of hypertensive men during short-term cold exposure. Am. J. Hypertens. 27, 656–664 (2014).
Burnett, R. W. & Itano, M. An interlaboratory study of blood-gas analysis: dependence of pO2 and pCO2 results on atmospheric pressure. Clin. Chem. 35, 1779–1781 (1989).
Smith, R., Edwards, P. & da Silva, A. Are Periods of Low Atmospheric Pressure Associated with an Increased Risk of Abdominal Aortic Aneurysm Rupture? Ann. R. Coll. Surg. Engl. 90, 389–393 (2008).
Edwin, F., Aniteye, E. A., Sereboe, L. & Frimpong-Boateng, K. eComment: Acute aortic dissection in the young–distinguishing precipitating from predisposing factors. Interact. Cardiovasc. Thorac. Surg. 9, 368 (2009).
Acknowledgements
There is no source of outside support for research, and no financial support by industry.
Author information
Authors and Affiliations
Contributions
G.G., S.N. and A.M.: collected the data. S.N. and PA.G.: performed the statistical analysis and prepared figures. S.N., G.G., J.S. and B.M.: wrote the main manuscript. PA.G., T.S. and P.D.: designed the study. All the authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
G.G., S.N., A.M., T.S., P.D., PA.G.: no conflict of interest. J.S.: Consultant speaker and proctoring fees from Cook Medical, consultant and speaker fees from Abott, consultant fees from GE, travel expenses from WL Gore, research grant from WL Gore and Cook Medical. B.M.: Consultant speaker and proctoring fees from Cook Medical, speaker fees from Philips.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guimbretière, G., Nusinovici, S., Monnot, A. et al. Impact of Relative Change in Temperature and Atmospheric Pressure on Acute Aortic Syndrome Occurrence in France. Sci Rep 10, 76 (2020). https://doi.org/10.1038/s41598-019-56841-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56841-w
This article is cited by
-
Circulation weather types and hospital admissions for cardiovascular disease in Changchun, China
Environmental Geochemistry and Health (2022)
-
Do meteorological factors influence the occurrence of acute aortic dissection? A 10-year retrospective institutional study
General Thoracic and Cardiovascular Surgery (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.