Abstract
To investigate the differential expression of tear cytokine levels among chronic Stevens–Johnson syndrome (SJS) patients to better understand the role of significantly altered cytokines in disease development. Tear samples were collected using Schirmer strips in 24 eyes of chronic SJS, 24 eyes of age and gender-matched controls, and 14 eyes of aqueous deficiency dry eye disease (DED) patients. The cytokine analysis was performed among 18 analytes which include pro-inflammatory, anti-inflammatory factors, and ELR-negative CXC chemokines. String analysis was performed for the significantly altered cytokines to understand their co-expression and role in the disease development. Additionally, a literature review was conducted to identify the signature cytokines present in chronic SJS tears. The differential expression of IL-6 (p ≤ 0.029), CXCL8/IL-8 (p ≤ 0.009), IL-1β (p ≤ 0.041), IL-2 (p ≤ 0.025), IL-10 (p ≤ 0.053), and CXCL-10 (p ≤ 0.044) were observed in chronic SJS patients and healthy controls. Whereas, IL-6 (p ≤ 0.029), CXCL8/IL-8 (p ≤ 0.058), CCL4 (p ≤ 0.056), GM-CSF (p ≤ 0.0001) IL-10 (p ≤ 0.025), and CXCL-10 (p ≤ 0.010), were differentially expressed in SJS as compared to severe DED patients. String analysis of the significantly altered cytokines revealed the involvement of several biological processes including the chronic inflammatory response, nitric oxide synthesis, angiogenesis, and cellular response to drugs. Among all the cytokines evaluated, the expression of CXCL8/IL-8 and CXCL10 levels were consistently reported in the literature. There was a differential expression of tear cytokines in SJS when compared to DED and healthy controls. The differential expression of CXCL8/IL-8 and CXCL10 was in line with existing literature and their role in chronic SJS pathogenesis merits further evaluation.
Similar content being viewed by others
Introduction
Stevens–Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) is a rare, acute, and serious, mucocutaneous drug reaction clinically characterised by blister formation and epithelial sloughing in the acute stage1. Even though the incidence of the disease ranges from 0.6 to 12 cases per million population per year in various populations, the severity of the disease can be extreme and therefore demands utmost and urgent care2. One of the well-known triggers of SJS is drug induced hypersensitivity whereas others are bacterial and viral infections3. According to a systematic review conducted in India, the most common drugs responsible for SJS were sulphonamides (37%), anti-epileptics (36%), and NSAIDS (16%) with an overall mortality of 13%. In a nine-year study, 19.5% of hospitalized patients with severe cutaneous adverse reactions in India were attributed to SJS. Therefore, it is widely speculated that the incidence of SJS could be higher in India as compared to the Western hemisphere4,5.
All the mucosal surfaces including ocular, genital, gastro-intestinal, nasal, renal, pulmonary, and buccal cavity can be involved at the disease onset6,7. However, the chronic sequelae of SJS are frequently ocular and include eyelid margin keratinization, meibomian gland dysfunction, conjunctival cicatrization and progressive corneal damage, which can be potentially blinding. Ocular surface dryness is another important consequence in SJS patients where the combined involvement of aqueous tear deficiency, decreased ocular surface wettability and an elevated rates of tear evaporation are observed8,9. At the cellular level there are distinct immunological responses occurring in SJS, which involves the secretion of cytokines, co-stimulatory and inflammatory molecules10. Hence, recent studies in the field of ocular inflammatory diseases have been focused on cytokine profiling of ocular fluids like tears and vitreous. Cytokine studies aid in the diagnosis of the disease and also for specific therapeutic management of the disease condition which are often associated with inflammation. For example, identification of these cytokines levels makes the classification of ocular allergic diseases possible11.
In the present study, we designed a customized panel to analyse the tears from chronic SJS patients for the expression of pro-inflammatory cytokines IL-1β, IL-2, IL-6, CXCL8/IL-8, IL-15,IL-17A, bFGF, RANTES, MCP-1, GM-CSF, TNF-α, IFN-γ and anti-inflammatory cytokines IL-10, IL-13 along with ELR-negative CXC chemokines CXCL9 and CXCL10. We explored and analysed the tears of Indian patients reporting to a tertiary eye centre and looked for differential expression of our designed panel of cytokines and compared with the healthy controls and severe dry eye disease (DED) patients. We also performed String analysis which helped to explore various plausible biological processes that could be linked with altered cytokines in chronic SJS tear samples. We believe our study findings will be helpful in better understanding of the SJS disease process in the eye and lead to insights that may eventually lead to better management and improvement in treatment outcomes.
Results
In this study, SJS patients (n = 12, 24 eyes; 7 males/5 females; mean age: 32.25 ± 12.12 years) (as shown in Fig. 1), healthy controls (n = 12, 24 eyes; 7 males/5 females; mean age: 30.8 ± 10.34 years) and non-SJS aqueous deficiency DED patients (n = 7, 14 eyes; 2 males/5 females; mean age: 43.5 ± 12.73 years) were recruited. The controls were age-matched/gender-matched volunteers and the tear collection time and total protein concentration in controls, SJS and DED are given in Table 1. Additionally patient details including age, gender, duration since onset of drug reaction, drug affected with and clinical symptoms examined under slit lamp microscope were detailed in Table 2. The data analysis with respect to the usage of topical steroids/anti-inflammatory drugs showed patients who are not under topical steroids have down-regulation of CXCL10 (p value ≤ 0.06) and IL-10 (p value ≤ 0.042) compared to patients who are on topical steroids/anti-inflammatory drugs. Simultaneously, the pro-inflammatory factors IL-2 (p value ≤ 0.04) and bFGF (p value ≤ 0.05) were upregulated in patients who are not on topical steroids/anti-inflammatory drugs (the bilateral usage of topical steroids and its dosage is given in the Supplementary Table S1). On further analysis of gender based effects in the SJS group, CXCL9 (p value ≤ 0.05) and IL-15 (p value ≤ 0.02) were significantly upregulated in females, otherwise none of the cytokines in the panel has shown any gender bias.
Ocular characteristics in eyes with chronic Stevens–Johnson syndrome. (A) Lid margin keratinization with tarsal conjunctival scarring in the upper eyelid (patient 11); (B) Upper lid post lid margin mucous membrane graft (patient 9); (C) peripheral corneal vascularization (patient 11); (D) Corneal scarring with vascularization with peripheral limbal vascularization (patient 9).
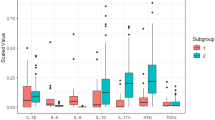
Pro-inflammatory cytokines in chronic SJS tears
We observed significant upregulation of pro-inflammatory cytokines IL-6 (p-value ≤ 0.029), CXCL8/IL-8 (p-value ≤ 0.009), IL-1β (p-value ≤ 0.041) and IL-2 (p-value ≤ 0.025) in tears of chronic SJS patients when compared to controls. There is no significant difference found in the levels of TNF-α, IFN-γ, CCL2, CCL5, CCL4, IL-17A, GM-CSF, FGF-basic, IL-15 and CCL11 in tears of control vs SJS patients. The comparative analysis of the tear samples in chronic SJS patients with DED patients revealed that IL-6 (p-value ≤ 0.015) was significantly upregulated, simultaneously, GM-CSF (p-value ≤ 0.0001) was highly downregulated (Fig. 2). There was no significant differential expression of CXCL8/IL-8, CCL4, TNF-α, CCL2, IL-1β, IL-17, IL-2, IFN-γ, IL-15, IL-17A, FGF-basic and CCL11 among severe DED and chronic SJS tears.
Anti-inflammatory cytokines in chronic SJS tears
The anti-inflammatory cytokine IL-10 (p-value ≤ 0.053) was found to be downregulated but not statistically significant in Chronic SJS tears when compared with controls. The comparative analysis of IL-10 (p-value ≤ 0.025) in SJS tears with severe DED was significantly downregulated (Fig. 2) and IL-13 didn’t exhibit any significant difference in chronic SJS tears compared to controls and severe DED.
ELR-negative CXC chemokines in chronic SJS tears
Among the two ELR-negative CXC chemokines in this study, CXCL10 was significantly downregulated when compared with healthy controls (p value ≤ 0.044). The CXCL10 levels in the SJS patients (p value ≤ 0.010) were significantly downregulated when compared with severe DED tears. Whereas, CXCL9 didn’t show any significant change in SJS patients in comparison with controls and severe DED tears (Fig. 2).
String analysis
The co-expression analysis between 18 analytes revealed the RNA co-expression patterns, and protein co-regulation in the homeostatic conditions (Fig. 3). The scores of CXCL9 and CXCL10 were observed to be 0.848, which was the highest among all the analytes. IL-6 and CXCL8/IL-8 showed the second highest RNA co-expression score of 0.581. String analysis revealed the protein–protein interactions between nine selected proteins i.e., IL-1β, IL-2, IL-6, CXCL8/IL-8, IL-10, TNF-α, IFN-γ, CXCL9, CXCL10 (Fig. 4). The network statistics were 9 number of nodes, 36 number of edges and PPI enrichment value was 2.51e−10. Various biological processes are found to be involved in the development of chronic SJS which includes nitric oxide biosynthesis, regulation of apoptotic processes, response to chronic inflammation, defence response and the cellular response to the drug. The cytokines involved in the particular process has been tabulated for better understanding (Table 3).
Above data was generated using String software analysis from the existing literature. The figure illustrates the co-expression of the cytokines in normal homeostatic conditions which are reported in this study, where it is found that CXCL10 and CXCL9 are highly co-expressive (RNA co-expression score 0.848). And the next highest co-expressive chemokines are CXCL8/IL-8 and IL-6 which are pro inflammatory cytokines (RNA co-expression score 0.581). The results obtained in the current study reveals that the co-expression of CXCL10 and CXCL9 was lost in chronic SJS patient tear.
The above figure shows the protein–protein interactions and its network statistics of the significantly altered cytokines in the current study, among chronic SJS patients when compared with normal and the cytokines involved in specific biological processes are detailed in Table 3.
Comparison of cytokine studies in chronic SJS tears
Till now, there are three reports from distinct groups that studied the tear cytokine levels in chronic SJS; one study from India, and other two from Japan. These studies have analysed different cytokine levels in 50–155 eyes. Gurumurthy et al. reported the significantly altered cytokines involved in chronic SJS tears, in response to lid margin keratinization, among pre and post mucous membrane grafting (MMG)12. Ueta et al. had compared the cytokines between atopic keratoconjunctivitis and chronic SJS13 and recently the same group (Yoshikawa et al.) had updated that CXCL8/IL-8 and IP-10 was involved in conjunctivalization and neovascularization respectively, while GrzB has role in keratinization by correlating with the clinical parameters from Ocular surface grading in chronic SJS patients14. The comparative analysis among the four studies including this had revealed that CXCL8/IL-8 and CXCL10 are commonly identified among inflammatory factors in all the studies (Table 4). Similarly, CXCL8/IL-8 levels were shown to be upregulated and IP-10/CXCL-10 were downregulated in all the four studies. These can be considered to be the common signature cytokine pattern in Chronic SJS ocular surface.
Discussion
This study tried to explore and discern the differential expression of various pro- and anti-inflammatory cytokines and chemokines in the tear film and ocular surface of patients with chronic ocular complications of SJS. We compared our findings with age and gender-matched healthy controls and patients with aqueous deficiency DED. Additionally, we compared our findings with the results of other similar studies in which the tear cytokine profile in chronic SJS has been reported (Table 3). The probable reasons for the differential regulation and their possible inter-relationship in the development of ocular disease in SJS have been unravelled but needs further validation in future studies.
Stevens–Johnson syndrome is an immune-mediated disorder, in the chronic sequelae, ocular surface is destroyed (as shown in Fig. 1) due to the damage that occurred in the acute stage15,16. Even though there are various inflammatory cytokines reported to understand the etio-pathogenesis of SJS, still there is a lack in identifying the signature inflammatory factor for the diagnosis and treatment of SJS. The common biological pathways and its interaction with the inflammatory factors need to be established to uncover the pathogenesis of SJS. Our study shows similar trends with respect to expression pattern of inflammatory cytokines studied previously in chronic SJS patients but differ with the level of significance of IL-10, IL-15, IL-17A, Bfgf, RANTES/CCL5, MCP-1, GMCSF, MIP1B/CCL4, TNF-α, and IFN-γ12. Also, we couldn’t find any significant difference among pre-MMG and post-MMG as reported in Gurumurthy et al., because of the modest sample size (3 post MMG patients). The reason behind selecting CXCL9 along with CXCL10 among ELR-negative CXC chemokines is because of their co-expression and similar intracellular domains for the activation of CXCR317. And to check if they are co-expressing in chronic SJS ocular surface or not, but interestingly they are not co-expressive. CXCL10 was significantly downregulated and CXCL9 doesn’t exhibit any difference in chronic SJS tears when compared to normal and patients with severe DED.
In the current study we also used String, a bioinformatic tool for analysis of the significantly altered cytokines belonging to pro-inflammatory cytokines, anti-inflammatory cytokines, and ELR-negative CXC chemokines. The significantly altered cytokines in chronic SJS with healthy controls and severe DED were selected for understanding the role of each cytokine in biological pathways, the String analysis was utilized. The String analysis of significantly altered cytokines has shown that TNF-α and IL-10 are involved in the chronic inflammatory response in chronic SJS tears. It has been previously reported that nitric oxide levels were significantly increased in the serum of acute SJS patients18. This study has been helpful in understanding that, among the differentially regulated cytokines in chronic SJS, IFN-γ, IL-1β and TNF-α were significantly involved in the regulation of nitric oxide biosynthesis. However, the String analysis has shown that IL-1β, 6, 8, 10 and CXCL10 were involved in the biological process in regulation of angiogenesis. We still need to consider the limitation of all the data base on which String analysis do prediction using inbuilt algorithms and it aims to track only all available protein association.
It has been reported earlier that CXC chemokines that contains the ELR motif are potent promoters of angiogenesis and those that lack ELR are potent inhibitors of angiogenesis19. This difference in angiogenic activity may impact the pathogenesis of variety of disorders. CXCL8/IL-8 is an ELR motif-containing cytokine, which is highly expressing in the ocular surface of chronic SJS can be the promoter of angiogenesis in SJS. In the same way CXCL10, ELR-negative chemokine is an inhibitor of angiogenesis is down regulated in SJS. Simultaneously, the imbalanced expression of CXCL8/IL-8 and CXCL10 were also observed in other fibro-proliferative disorders i.e., Idiopathic pulmonary fibrosis (IPF)20. The exogenous IP‐10 (intramuscular) was administered in the bleomycin‐induced pulmonary fibrosis murine model, which resulted in marked attenuation of pulmonary fibrosis that was entirely attributable to a reduction in angiogenesis in the lung21.
On the flip side, among the co-expression of these analytes, CXCL8/IL-8 and IL-6 were found to be co-expressive and are capable of highly expressing pro-inflammatory cytokines in SJS tears. While CXCL10 and CXCL9 were highly co-expressive among all cytokines in our panel, but in SJS tears they were not consistent in terms of co-expression. We identified that CXCL10 levels are downregulated in the chronic SJS tear, unlike CXCL9 which has no significant difference in terms of expression even though they are highly co-expressive, in homeostatic conditions. The difference in the expression pattern of ELR-negative CXC chemokines in SJS might be because of the different effects of ligands on the variants of its receptors17. Also, these ligands have different temporal and spatial expressions in distinct cell types. However, these three ligands activate the same receptor but exhibit unique and differential expression which has been reported in various diseases like psoriasis22.
The main limitations of our study were the modest sample size and inability to correlate the ocular cytokine expression with the systemic level inflammatory factors. Since SJS is an extremely rare disease, the limitation in sample size is expected. Additionally, Out of 12 patients in the study, 11 had similar grading for eyelid, conjunctival, and corneal findings in both eyes, thus reflecting similarity in the magnitude of chronic ocular complications bilaterally (Table 2). Since most patients had severe dry eye as a component of the severe ocular complications, we did not get enough yield for the tear samples, and hence had to pool the tear samples from both eyes of the same patient. Therefore, we were unable to study the potential variability between eyes which might provide better insight of disease pathophysiology.
However, the study also has significant strengths including the comparison with similar ocular surface inflammatory condition i.e., severe DED tears, String analysis and a literature review to compare the expression profiles across different studies.
In conclusion, this study investigated the differential expression of tear cytokine levels among chronic SJS patients to understand the role of significantly altered cytokines in disease development. The study found that there was a differential expression of tear cytokines in SJS when compared to DED and healthy controls. The differential expression of CXCL8/IL-8 and CXCL10 was in line with existing literature and the role of these specific cytokines in chronic SJS pathogenesis merits further evaluation.
Materials and methods
Tear samples were collected from 24 eyes (n = 12) of SJS/TEN patients, 36 eyes (n = 12) of age and gender-matched healthy controls, and 14 eyes (n = 7) of severe DED patients during the period of 2018 to 2019. The study protocol was approved by the institutional review board of LVPEI ethics committee and were performed under the tenets of the declaration of Helsinki. All the participants in this study had provided written informed consent before enrolling in this study. Written informed consent was obtained from all individuals for publication of images which were obtained from the electronic medical records. The demographics of participants of the study are detailed in the Table 1. The clinically diagnosed chronic SJS patients with a history of more than one year of onset of the reaction were recruited in this study. The diagnosis of SJS was confirmed based on the history of acute reaction in the skin and the mucous membrane involvement23. The criteria used to define severe dry eye was Schirmer score < 10 mm, OSDI score > 13 and positive corneal staining (> 1 on Oxford Staining Score (OSS))24.
Tear collection and storage
The tear sample were collected from each eye of chronic SJS patients by performing Schirmer test 1 (without topical anesthesia) and the same has been followed in controls and severe dry eye disease (DED) patients. Initially Schirmer strip was placed in the cul-de-sac of the eye for 5 min (in case of controls, till it reaches 30 mm). These Schirmer strips with tears in it were placed under sterile conditions in a 500 µL punctured centrifuge tubes. The punctured 500 µL tubes were placed in 1.5 mL sterile centrifuge tubes as reported previously (Posa et al.)25 and samples were collected by centrifugation at 13,000 rpm for 5 min and immediately stored at − 80 °C until further use. The protein concentrations were estimated using the BCA method (Cat #786-570, G-Biosciences, Geno Technology Inc., USA).
Estimation of tear cytokines using Luminex assay
The concentration of eighteen analytes were estimated using a customized panel of Luminex ELISA (LXSAHM-18, USA R&D Systems, Inc). The list of analytes measured were CCL2/JE/MCP-1, CCL4/MIP-1 beta, CCL5/RANTES, CCL11/Eotaxin, CXCL9/MIG, CXCL10/Interferon-γ induced protein-10/CRG-2, Fibroblast Growth Factor-basic/FGF2/bFGF, Granulocyte Monocyte-Colony Stimulating Factor, Interferon-γ, IL-1 β/IL-1F2, IL-2, IL-6, CXCL8/IL-8, IL-10, Interleukin-13, Interleukin-15, Interleukin-17 and tumour necrosis factor-α. All the reagents were allowed to obtain to room temperature (RT) prior to the experiments. The reagents were prepared according to the manufacturer’s guidelines. The total sample volume was adjusted to 35 µL with 150 mg/mL of total protein concentration. After the normalization of samples and preparation of standards, 50 µL of standards and 35 µL of the sample was added in appropriate wells as labelled. To these wells, 50 µL of diluted microparticle cocktail consisting of 18 analytes were added and followed by incubation for 2 h at room temperature on a shaker at 800 rpm. After incubation, the wells were washed thrice with 100 µL of wash buffer followed by incubation with 50 µL of diluted biotin-antibody cocktail for 1 h at RT on the shaker at 800 rpm. After incubation, 50 µL of diluted streptavidin-PE was added to each well and incubated for 30 min at room temperature at 800 rpm. Finally, the assay plate was decanted and washed 3 times with 100 µL of wash buffer. In the end, 150 µL of wash buffer was added and incubated for 2 min at RT on the shaker at 800 rpm and then read on a Luminex reader by using XPONENT 4.2 software using default parameters and results were obtained in Median fluorescent intensity (MFI), which were further used to analyse the data.
String analysis
The String (Search Tool for Retrieval of Interacting Genes/Proteins) biological database (version 11), was used to perform the co-expression analysis for better understanding of the RNA co-expression patterns and protein co-regulation in SJS patients. The network type was indicating the edges with both functional and physical protein associations. Default prediction methods were used with high confidence scores at > 0.7. The analytes given to the String database were gene names of all the analytes in our customized panel i.e., IL1β, CCL2, CCL4, CCL5, CCL11, CXCL9, CXCL10, FGF, GMCSF, IFN-γ, IL2, IL6, IL8/CXCL8, IL10, IL13, IL15, IL17 and TNF-α. The heat map was generated using the software, giving the RNA co-expression patterns, and protein co-regulation provided by ProteomeHD in String database.
The protein–protein interactions were established for the significantly altered inflammatory cytokines IL6, IL1β, IL8, IL10, IL2, two anti-fibrotic cytokines TNF-α, IFN-γ and ELR-negative CXC chemokines i.e., CXCL10 and CXCL9. The analysis of this protein–protein interaction network was used for better understanding of the roles of cytokine in a biological processes.
Comparison of cytokine studies in chronic SJS tears
To correlate the cytokine studies reported till now in Chronic SJS tears, we have done the PubMed search with the keywords, Cytokine/SJS/Chronic. PubMed displayed 21 search reports for the given keywords. Out of these, 3 studies were found to be similar to compare with our study in terms of sample type (tear), chronic stage patients, age group (adult) of the patient and the duration from onset of disease. All the analytes studied till now as mentioned in the above three studies are correlated with our study and has been tabulated. The significance has been compared between the selected studies for the cytokines in SJS and the healthy controls of the respective studies.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 in between the three groups SJS, Control, and severe DED. Data was expressed as the mean and evaluated with Student’s t-test. Graphical representation has been done by using whiskers Tukey. p-value ≤ 0.05 was considered as significant and ≤ 0.001 as highly significant.
References
Creamer, D. et al. U.K. guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. Br. J. Dermatol. 174, 1194–1227. https://doi.org/10.1111/bjd.14530 (2016).
Naldi, L., Locati, F., Marchesi, L. & Cainelli, T. Incidence of toxic epidermal necrolysis in Italy. Arch. Dermatol. 126, 1103–1104. https://doi.org/10.1001/archderm.1990.01670320127028 (1990).
Patel, T. K., Barvaliya, M. J., Sharma, D. & Tripathi, C. A systematic review of the drug-induced Stevens–Johnson syndrome and toxic epidermal necrolysis in Indian population. Indian J. Dermatol. Venereol. Leprol. 79, 389–398. https://doi.org/10.4103/0378-6323.110749 (2013).
Shanbhag, S. S. et al. Genetic markers for Stevens–Johnson syndrome/toxic epidermal necrolysis in the Asian Indian population: Implications on prevention. Front. Genet. 11, 607532. https://doi.org/10.3389/fgene.2020.607532 (2020).
Hazin, R., Ibrahimi, O. A., Hazin, M. I. & Kimyai-Asadi, A. Stevens–Johnson syndrome: Pathogenesis, diagnosis, and management. Ann. Med. 40, 129–138. https://doi.org/10.1080/07853890701753664 (2008).
Harr, T. & French, L. E. Toxic epidermal necrolysis and Stevens–Johnson syndrome. Orphanet J. Rare Dis. 5, 39. https://doi.org/10.1186/1750-1172-5-39 (2010).
Shanbhag, S. S. et al. Multidisciplinary care in Stevens–Johnson syndrome. Ther. Adv. Chronic Dis. 11, 2040622319894469. https://doi.org/10.1177/2040622319894469 (2020).
Sotozono, C., Ueta, M. & Yokoi, N. Severe dry eye with combined mechanisms is involved in the ocular sequelae of SJS/TEN at the chronic stage. Investig. Ophthalmol. Vis. Sci. 59, 80–86. https://doi.org/10.1167/iovs.18-24019 (2018).
Singh, S. et al. Lacrimal gland involvement in severe dry eyes after Stevens–Johnson syndrome. Ophthalmology 128, 621–624. https://doi.org/10.1016/j.ophtha.2020.08.016 (2021).
Abe, R. Immunological response in Stevens–Johnson syndrome and toxic epidermal necrolysis. J. Dermatol. 42, 42–48. https://doi.org/10.1111/1346-8138.12674 (2015).
Cook, E. B. Tear cytokines in acute and chronic ocular allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 4, 441–445. https://doi.org/10.1097/00130832-200410000-00018 (2004).
Gurumurthy, S., Iyer, G., Srinivasan, B., Agarwal, S. & Angayarkanni, N. Ocular surface cytokine profile in chronic Stevens–Johnson syndrome and its response to mucous membrane grafting for lid margin keratinisation. Br. J. Ophthalmol. 102, 169–176. https://doi.org/10.1136/bjophthalmol-2017-310373 (2018).
Ueta, M., Nishigaki, H., Sotozono, C. & Kinoshita, S. Downregulation of interferon-gamma-induced protein 10 in the tears of patients with Stevens–Johnson syndrome with severe ocular complications in the chronic stage. BMJ Open Ophthalmol. 1, e000073. https://doi.org/10.1136/bmjophth-2017-000073 (2017).
Yoshikawa, Y. et al. Predictive biomarkers for the progression of ocular complications in chronic Stevens–Johnson syndrome and toxic eeidermal necrolysis. Sci. Rep. 10, 18922. https://doi.org/10.1038/s41598-020-76064-8 (2020).
Basu, S. et al. Chronic ocular sequelae of Stevens–Johnson syndrome in children: Long-term impact of appropriate therapy on natural history of disease. Am. J. Ophthalmol. 189, 17–28. https://doi.org/10.1016/j.ajo.2018.01.028 (2018).
Singh, S. et al. Lid margin keratinization in Stevens–Johnson syndrome: Review of pathophysiology and histopathology. Ocul. Surf. https://doi.org/10.1016/j.jtos.2021.03.011 (2021).
Tokunaga, R. et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 63, 40–47. https://doi.org/10.1016/j.ctrv.2017.11.007 (2018).
Ivanyushko-Nazarko, N. V., Syzon, ОО, Volbyn, S. V., Rudnyk, T. I. & Dashko, M. O. Examining the role of the nitric oxide system as the essential pathogenetic link in Stevens-Johnson syndrome. Wiad. Lek. 73, 1900–1903 (2020).
Belperio, J. A. et al. CXC chemokines in angiogenesis. J. Leukoc. Biol. 68, 1–8 (2000).
Keane, M. P. et al. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J. Immunol. 159, 1437–1443 (1997).
Keane, M. P. et al. IFN-gamma-inducible protein-10 attenuates bleomycin-induced pulmonary fibrosis via inhibition of angiogenesis. J. Immunol. 163, 5686–5692 (1999).
Dufour, J. H. et al. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 168, 3195–3204. https://doi.org/10.4049/jimmunol.168.7.3195 (2002).
Shanbhag, S. S. et al. Clinical clues predictive of Stevens–Johnson syndrome as the cause of chronic cicatrising conjunctivitis. Br. J. Ophthalmol. 104, 1005–1009. https://doi.org/10.1136/bjophthalmol-2019-314928 (2020).
Singh, S., Shanbhag, S. S. & Basu, S. Tear secretion from the lacrimal gland: Variations in normal versus dry eyes. Br. J. Ophthalmol. https://doi.org/10.1136/bjophthalmol-2020-318159 (2021).
Posa, A. et al. Schirmer strip vs capillary tube method: Non-invasive methods of obtaining proteins from tear fluid. Ann. Anat. 195, 137–142. https://doi.org/10.1016/j.aanat.2012.10.001 (2013).
Sotozono, C. et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens–Johnson syndrome. Ophthalmology 114, 1294–1302 (2007).
Acknowledgements
Supported by the Hyderabad Eye Research Foundation, Hyderabad, India. We would like to express special thanks of gratitude to all the patients who are involved in this study.
Author information
Authors and Affiliations
Contributions
Conception and design: K.M.A., S.S., V.S. and S.B. Analysis and interpretation: K.M.A., D.P., S.U., J.J., S.S., S.B. and V.S. Data collection: K.M.A., D.P., S.U., J.J. and S.S. Supervision and direction: S.S., S.B. and V.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koduri, M.A., Prasad, D., Upadhyaya, S. et al. Differential expression of tear film cytokines in Stevens–Johnson syndrome patients and comparative review of literature. Sci Rep 11, 18433 (2021). https://doi.org/10.1038/s41598-021-97575-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97575-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.