Abstract
Study design:
This was a prospective cohort study.
Objectives:
The objective was to describe the incidence, prevalence, characteristics of pressure ulcers (PUs) and the association with specific patient characteristics in a consecutive sample of in-patients with a spinal cord injury (SCI).
Setting:
An acute care and rehabilitation clinic specialized in SCIs in Switzerland.
Methods:
The presence and characteristics of PUs for all adult patients with a SCI admitted to the clinic from 1 September 2009 to 28 February 2010 were recorded on a daily basis during their complete hospitalization. Risk factors were analyzed in univariate and multivariate logistic regression models.
Results:
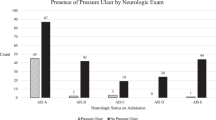
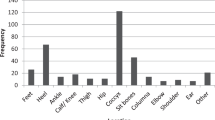
A total of 185 patients were included in the study and observed for the entirety of their hospitalization. The prevalence of at least one PU was 49.2% in all patients, compared with 25.4% in the group of patients admitted without PUs. The incidence was 2.2 per person and year. In 91 patients, a total of 219 PUs were observed. PUs were most frequently located on the foot (36.1%), and the coccyx/sacrum (15.1%). The risk for occurrence of a PU increased with age (odds ratio (OR)=1.04) and post SCI (OR=1.03). In the multivariate analyses, the risk for PUs was lower for patients with the American Spinal Injury Association (ASIA) Impairment Scale (AIS) of C or D (ORC=0.25, ORD=0.28) compared with patients with an AIS of A.
Conclusion:
Using a daily documentation system, PUs were detected as a frequent complication of SCIs. Completeness of injury, age and time since injury were significant risk factors for PUs. The foot was a region at high risk for PUs.
Similar content being viewed by others
Introduction
Pressure ulcers (PUs) are the second most common comorbid condition in individuals with a spinal cord injury (SCI);1, 2, 3 85% of individuals with a SCI develop a PU at least once during their lifetime.4, 5 A PU is a challenge for SCI patients as it has many consequences such as immobilization, which leads to lengthened hospital stays, and can influence the quality of life dramatically.4, 6, 7 Not only are PUs the second most frequent reason for rehospitalization in the SCI population, but they also cause the highest costs, up to billions of dollars.4, 5, 8, 9
Retrospective studies of PUs among SCI in-patients found prevalence ranging up to 69.2% in the early 1980s,10 and between 31.5 and 56% nowadays.2, 6 One prospective cohort study decribed a prevalence of 36.5% during the acute phase, and 39.4% during the initial rehabilitation.7 However, although there has been a great deal of research on PU prevalence and associated risk factors, many previous studies were prone to bias because of study design and collection methods that may have overestimated or underestimated the prevalence of PUs. For example, information may get lost when self-report questionnaires and patients records are used and data are collected retrospectively. In addition, grade 1 PUs, defined as nonblanchable redness without a loss of the epidermis, might be reported more often with a higher quality of observation and documentation.
The aim of this study was to gain more knowledge of the characteristics of PUs in adult SCI patients during their stay in the Swiss Paraplegic Centre (SPZ) by: (1) evaluating the incidence and prevalence of PUs among in-patients, (2) describing the location and grade of PUs and by (3) determining the association between specific patient characteristics and the incidence of a PU.
Materials and methods
Patients and setting
Data were collected at the SPZ in Nottwil, Switzerland. The SPZ is an acute care and rehabilitation clinic, and includes an intensive care unit specialized in SCI. The patient population includes those admitted for acute treatment of SCI, initial rehabilitation, re-rehabilitation because of complications such as urologic or orthopedic surgery and ventilation-dependent patients. Patients are treated by an interdisciplinary team and paraplegia specialists (PMR) following the recommendation of the SCIRE project12 and European Pressure Ulcer Advisory Panel (EPUAP).13
We included all patients with a SCI admitted to the hospital between 1 September 2009 and 28 February 2010 (6 months) for in-patient treatment, who were at least 18 years old and were classified AIS A–D according to the American Spinal Injury Association (ASIA) Impairment Scale (AIS). There were no exclusion criteria.
Procedures
Measurements were systematically recorded starting at a patient’s admission until their discharge. In order to limit potential sources of bias, missing data and other data quality issues, we applied, in a prospective cohort study, a daily documentation system. The documentation sheet covered sociodemographics (that is, age and gender), dates and reason of hospitalization, SCI-specific data (that is, neurologic level of injury, AIS at admission and date and cause of SCI) as well as PU characteristics (that is, grade, location and date of first occurrence, and change or healing in PU). Information on surgical treatment of the PU (yes/no) was also gathered. A PU grade was defined using the classification by Daniel et al.14 that was used as a standard in the hospital and consists of five grades of severity (based on tissue damage and depth) (Table 1).
During this period, the skin status of every patient was assessed twice a day by the nursing staff and documented. Follow-up time for each PU was completed at healing of the PU or censored at discharge of the patient.
All data were recorded on a standardized documentation sheet. Before the study, the responsible nursing staff was trained on how to assess and document PUs and was supported at all times by a supervising nurse. After a patient’s discharge, all data were transferred by the supervising nurse from a paper version into a database. Data were checked for plausibility and missing values were corrected by the supervising nurse, if needed.
Analysis
Descriptive statistics were used for patient and PU characteristics. The distribution of grades (at first observation) and locations of PUs were described using frequencies, and totals were given including and excluding PUs grade 1. Incidences were based on the number of individuals acquiring (one or several) new PUs during their complete hospitalization. The nonparametric Mann–Whitney U-test was used to compare length of stay. Univariate logistic regressions were applied to quantify the associations between risk factors and the occurrence or development of a PU. Variables were then entered into a multivariate logistic regression, using the enter method.
All analyses were done using PASW Statistics 18 (SPSS Inc., Chicago, IL, USA) and R, version 2.15.0 (R Development Core Team, Nottwil, Switzerland).
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research. The study protocol was approved by the local ethical committee of Lucerne (registration number 11095).
Results
A total of 185 patients admitted during the 6-month period were included. Their characteristics are presented in Table 2. No one was excluded and follow-up was 100%. It took 15.5 months from the start of inclusion until all included patients were discharged (1 September 2009 to 15 December 2010). The median length of stay was 51 days (interquartile range: 13–121 days). In total, 219 PUs were observed during the study time.
Incidence and prevalence of PU
Because of the occurrence of several PUs in the same patient at different locations and times, a complex course of PUs during hospitalization became apparent (Table 3). Out of 126 patients who had no PU at admission, 32 developed at least one PU during their hospital stay. Out of 59 patients admitted with at least one PU, 23 developed additional PUs during their hospital stay. The 25 patients discharged without complete healing of their PU had comorbidities including diabetes, psychiatric disorders or malignant diseases with poor prognosis.
Nearly half of the study population (n=91; 49.2%) had PUs during hospitalization (including grade 1). Sixteen patients (8.6%) had only grade 1 PUs. After excluding these patients, the percentage of patients with a PU decreased to 40.5% (n=75).
The incidence for a new PU (including all grades) in our population was 2.2 PU per person-year (PY). In other terms, the incidence was 1.8 PUs per 10 person-months. Excluding grade 1 PUs, the incidence decreased to 1.5 PU per PY. Considering only the 126 patients who did not have a PU at admission, the incidence for a new PU was 2.0 per PY (including all grades), and 1.3 per PY without grade 1 PUs. Of those patients who had a PU at admission (n=59), the incidence of another PU was 2.5 per PY, and 1.5 per PY without grade 1 PUs.
Location and grades of PU
Most of the patients with a PU (n=74; 81.3%) had 1–3 PUs, with a maximum of eight PUs found in one patient. In the case of more than one PU, they were generally locally clustered.
Two-thirds of the 219 PUs were grade 1 or 2. Severe PUs of grade 4 or 5 occurred in only 9.6% (n=21) of the patients (Table 4); none of those PUs were hospital acquired. More than half of the 106 in-house-developed PUs were grade 2, 34.9% were grade 1 (n=37) and only 11.3% of the PUs were grade 3 (n=12).
Risk factors
Performing (univariate and multivariate) logistic regressions for the presence of a PU with the available risk factors, three variables were significantly associated: age at admission, time since lesion and AIS (Tables 5 and 6 ).
A univariate logistic regression for the in-house development of a PU with the same risk factors, expanded by presence of a PU at admission, found only reason of admission to have a significant association (Table 7). After inclusion of all these risk factors into the multivariate model, time since lesion and reason of admission remained significant (Table 8). Patients affected the most by PUs were those admitted for initial rehabilitation (Table 9).
The median length of stay was compared between patients with a PU and those without one. Patients with a PU had a 5.1 time longer stay in the hospital compared with patients with no PU (Mann–Whitney U-test: P<0.001). The respective medians and interquartile ranges were 18 and 6–61 days for patients without PU and 92 and 44–153 days for patients with PUs.
Discussion
Through the use of a daily documentation system we detected a high incidence and prevalence of PUs in patients with SCI, especially of grade 1 and grade 2 PUs. The interaction of location, grade and patient characteristics, as well as the fact that several PUs can occur in one patient, confirmed PUs as a complex health condition.
Characteristics of the population
Our consecutive sample was comparable to existing studies on PUs in SCI in-patients concerning sample size, gender, etiology, age at onset of SCI and age at admission.5, 6, 7, 15 The percentage of patients with AIS A in our sample (65.4%) was higher than in other studies (11.2–54.0%).2, 5, 6, 7
Prevalence of PU
As previously mentioned, the prevalence of PUs has decreased over the past few decades. For example, a retrospective study using patient records from the 1980s reported a prevalence of 69.2%.10 Since then, care concepts have been developed, and more knowledge and technical devices are available today; recently reported prevalences of PUs range between 32 and 56%;2, 5, 6, 7, 16 when excluding grade 1, the prevalences range between 24 and 39%.2, 6, 7 In our study, which is based on a daily documentation protocol, the overall prevalence of PU was 49.2 and 40.5% when excluding grade 1 PU. Therefore, our reported prevalence of PUs was in the upper range of results from previous studies. However, results from different studies should be compared with caution because of the variation in study populations, health conditions and assessment methods. Our population was different compared with most studies on PUs because of variation in major risk factors such as completeness of lesion, age and time since SCI, which are known to be related with a higher incidence of PU. In addition, patients in the acute care phase, which were included in our study, often have surgeries and need intensive care and are also at a higher risk for a PU.17 Because of the amount of relevant health issues in patients with a SCI,18, 19 the protection and care of the skin has to be feasible and integrated in the complex treatment concept.2, 7, 12, 16 Although we found a significant lengthening of stay in patients with a PU, this difference has to be discussed carefully. PUs often occur in more critically ill patients and other reasons may interfere.
Location and grades of PU
The most frequent location of PU in our study was at the foot. Other studies found the highest incidence of PU at the sacrum.2, 3, 5, 6, 7 This difference may be explained by the retrospective data collection in the other studies. A PU at the seating region often leads to immobilization,2, 7 whereas PUs on the foot can heal spontaneously and hence might be underreported. In our study, as well as other studies, the most severe PUs (grades 4 and 5) were community acquired, and were localized over the sacrum, ischium, lower extremity and Trochanter.2, 9
Risk factors
Age at admission, time since SCI and completeness of lesion were significant risk factors for PUs in our study, which is similar to previous literature.4, 6, 8, 17 However, some risk factors reported as being significant in previous studies were not supported by our results: (non-) traumatic etiology or level of lesion.5, 7
In addition, because the time variables were entered in years, the risk for a PU hardly increases when comparing individuals differing by only 1 year in age or time since lesion. However, the P-value and the confidence intervals (one is not included) indicate there is a significant risk adding up with increasing years. This is important as SCI care is designed for a lifetime.
Clinical relevance
Although treatment has been optimized, following the recommendation of the SCIRE project12 and EPUAP,13 through the adaptation of medical and care concepts, training nurses, using technical devices and so on, the incidence of PU in a specialized clinic for SCI still remains high. At this point, more than 200 risk factors are known8, 13 and specialized risk assessment scales are used, but this may not be enough to reduce incidence rates.20 It seems that the precautionary awareness of caregivers and patients in different, rapidly changing health conditions is the key to reducing the incidence of PUs, and therefore needs further improvement. In order to achieve this, precise, daily observation and prompt documentation in a feasible reporting system on PUs (including grade 1) is necessary. Although this will probably result in higher incidence rates, consideration of these different aspects of PU incidence have to be used before thinking about the quality of care.17
In almost the same manner, intervention studies should be based on an appropriate reporting system and help to optimize care concepts. For example, the most frequent localization of PUs was at the foot, indicating that special devices and protection strategies focused on this body region are required for prevention.
Limitations
This study only covered the in-patient period and hence incidence of PU in the community is still unknown. In addition, the classification of PUs involves some overlap because of fluent transition of grades. As different nurses performed the assessments on PU severity, there could be variation in the judgment of PU grades. However, as the nursing staff was specially trained and had experience in applying the classification scale, we consider this unlikely in affecting our results. Finally, the study population consisted of only 185 patients, resulting in sometimes small subgroups and also only a subset of known risk factors. Therefore, to allow for generalization the sample size should be increased, observations from different centers be added and more relevant risk factors be tested.
Conclusion
PUs were detected as a frequent and complex complication of a SCI. We conclude that the dominant interactive factors in preventing and treating PUs are: general awareness, a precise daily observation and prompt documentation, knowledge of risk factors and a constantly improving care concept. A dynamic improvement process can be achieved through the use of a feasible daily documentation system on PUs as part of the clinical routine.
The use of incidences of PU in patients with SCI as an indicator for quality of care is only acceptable based on a good reporting system and considering general health conditions and specific risk factors. We found that although the quality of care is high in a hospital specialized in SCI, the risk for developing a PU in-house remains high because of inherent characteristics of the health conditions of SCI patients.
Data archiving
There were no data to deposit.
References
Krause JS, Carter RE, Pickelsimer EE, Wilson D . A prospective study of health and risk of mortality after spinal cord injury. Arch Phys Med Rehabil 2008; 89: 1482–1491.
New PW, Rawicki HB, Bailey MJ . Nontraumatic spinal cord injury rehabilitation: pressure ulcer patterns, prediction, and impact. Arch Phys Med Rehabil 2004; 85: 87–93.
Salzberg A . Predicting pressure ulcers during initial hospitalization for acute spinal cord injury. Wounds 1999; 11: 45–57.
Charlifue S, Jha A, Lammertse D . Aging with spinal cord injury. Phys Med Rehabil Clin N Am 2010; 21: 383–402.
Chen Y, Devivo MJ, Jackson AB . Pressure ulcer prevalence in people with spinal cord injury: age-period-duration effects. Arch Phys Med Rehabil 2005; 86: 1208–1213.
Ash D . An exploration of the occurrence of pressure ulcers in a British spinal injuries unit. J Clin Nurs 2002; 11: 470–478.
Verschueren JH, Post MW, de Groot S, van der Woude LH, van Asbeck FW, Rol M . Occurrence and predictors of pressure ulcers during primary in-patient spinal cord injury rehabilitation. Spinal Cord 2011; 49: 106–112.
Byrne DW, Salzberg CA . Major risk factors for pressure ulcers in the spinal cord disabled: a literature review. Spinal Cord 1996; 34: 255–263.
DeVivo M, Farris V . Causes and costs of unplanned hospitalizations among persons with spinal cord injury. Top Spinal Cord Inj Rehabil 2011; 16: 53–61.
Richardson RR, Meyer PR . Prevalence and incidence of pressure sores in acute spinal cord injuries. Paraplegia 1981; 19: 235–247.
Gelis A, Dupeyron A, Legros P, Benaim C, Pelissier J, Fattal C . Pressure ulcer risk factors in persons with spinal cord injury part 2: the chronic stage. Spinal Cord 2009; 47: 651–661.
SCIRE Spinal Cord Rehabilitation Evidence. Vancouver, BC: SCIRE Project/ Monkey Hill Health Communications, 2010 [cited 17 December 2012]; Available from http://www.scireproject.com.
European Pressure Ulcer Advisory Panel & National Pressure Ulcer Advisory Panel. Prevention and treatment of pressure ulcer; quick reference guide. National Pressure Ulcer Advisory Panel: Washington DC. 2009 [cited 30 November 2012]; Available from http://www.epuap.org/guidelines/QRG_Prevention_in_German.pdf.
Daniel RK, Hall EJ, MacLeod MK . Pressure sores-a reappraisal. Ann Plast Surg 1979; 3: 53–63.
Krause JS, Broderick L . Patterns of recurrent pressure ulcers after spinal cord injury: identification of risk and protective factors 5 or more years after onset. Arch Phys Med Rehabil 2004; 85: 1257–1264.
Gelis A, Dupeyron A, Legros P, Benaim C, Pelissier J, Fattal C . Pressure ulcer risk factors in persons with SCI: part I: acute and rehabilitation stages. Spinal Cord 2009; 47: 99–107.
Campbell C, Parish LC . The decubitus ulcer: facts and controversies. Clin Dermatol 2010; 28: 527–532.
Charlifue S, Lammertse DP, Adkins RH . Aging with spinal cord injury: changes in selected health indices and life satisfaction. Arch Phys Med Rehabil 2004; 85: 1848–1853.
Kirchberger I, Cieza A, Biering-Sorensen F, Baumberger M, Charlifue S, Post MW et al. ICF core sets for individuals with spinal cord injury in the early post-acute context. Spinal Cord 2010; 48: 297–304.
Kottner J, Dassen T, Tannen A . Inter- and intrarater reliability of the Waterlow pressure sore risk scale: a systematic review. Int J Nurs Stud 2009; 46: 369–379.
Acknowledgements
We thank all the professionals at the SPZ for contributing to the data collection and Karin Gläsche as the supervising and main responsible nurse. We also thank Jonviea Chamberlain for rereading and correcting the English language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Scheel-Sailer, A., Wyss, A., Boldt, C. et al. Prevalence, location, grade of pressure ulcers and association with specific patient characteristics in adult spinal cord injury patients during the hospital stay: a prospective cohort study. Spinal Cord 51, 828–833 (2013). https://doi.org/10.1038/sc.2013.91
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2013.91
Keywords
This article is cited by
-
Predictors of hospital-acquired pressure injuries in patients with complete spinal cord injury: a retrospective case–control study
BMC Musculoskeletal Disorders (2023)
-
Unplanned hospital admissions due to secondary health conditions after spinal cord injury: a population-based description of determinants of length of stay
Spinal Cord (2023)
-
Risk constellation of hospital acquired pressure injuries in patients with a spinal cord injury/ disorder - focus on time since spinal cord injury/ disorder and patients’ age
Spinal Cord (2023)
-
Incidence, severity and time course of pressure injuries over the first two years following discharge from hospital in people with spinal cord injuries in Bangladesh
Spinal Cord (2022)
-
Development of the spinal cord injury pressure sore onset risk screening (SCI-PreSORS) instrument: a pressure injury risk decision tree for spinal cord injury rehabilitation
Spinal Cord (2021)