Key Points
-
Reviews the research to date in the field of dental stem cells with an indication of where the field might be headed and the new directions in clinical applications.
-
Provides an overview of the types of dental stem cells that can be isolated from the dental and gingival tissues.
-
Highlights that dental and gingival stem cells are easily accessible during a routine extraction procedure in the dental clinic.
Abstract
Mesenchymal stem cells can be obtained with ease from dental/oral tissue, making them an attractive source of autologous stem cells. They offer a biological solution for restoring damaged dental tissues such as vital pulp engineering, regeneration of periodontal ligament lost in periodontal disease, and for generation of complete or partial tooth structures to form biological implants. Dental mesenchymal stem cells share properties with mesenchymal stem cells from bone marrow and there is a considerable potential for these cells to be used in different stem-cell-based therapies, such as bone and muscle regeneration. In addition, their immunosuppressive-immunomodulatory properties make these cells a suitable source for treating immunodisorders like systematic lupus erythematosus. In addition, gingival tissue might also be a very good source of epithelial cells used in the treatment of severe ocular surface disorders. Being such an accessible source for different stem cells, the tooth and the attached gingival tissue (usually discarded in the clinics) represent an ideal source of autologous or allogeneic stem cells that can be used in the treatment of many clinical conditions in dentistry and medicine.
Similar content being viewed by others
Introduction
Undifferentiated cells that have the potential to develop into specialised cell types that carry out different functions are generally described as 'stem cells' and defined biologically according to certain criteria such as the ability to self-renew or differentiate into multiple cell types. Stem cells from an adult tissue exist either in the context of organ and tissue growth homeostasis, or to provide a source of cells for repair.
The isolation and characterisation of mesenchymal stem cells from such an accessible source as the teeth has opened up a new field of research and the possibility of finding a source of autologous or allogeneic mesenchymal stem cells that can be used in the treatment of many clinical conditions in dentistry and medicine.
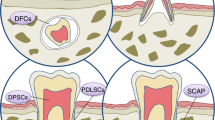
The sources to obtain different dental stem cell populations are commonly discarded extracted teeth and the attached tissue. The stem cell population of deciduous teeth can be obtained from the 20 milk teeth that are naturally replaced. The populations of dental pulp (DP) stem cells are obtained from dental pulp tissue, while the periodontal ligament (PDL) stem cells are obtained by scraping the mid third of the root in extracted teeth. The stem cells from the apical papilla (SCAP), are isolated from the apical papilla tissue and can easily be removed from an extracted tooth with still developing roots (very common in third molar extractions), a source for obtaining the stem cells from the dental follicle that surrounds the developing molar. The gingival stem cells (fibroblasts and epithelial) are easily isolated by simple biopsy or attached gingival tissue during the extractions. Once the tissue is obtained in the clinic, the isolation and expansion of the cells is done in a laboratory by setting up cell cultures using enzyme treatment and/or explants techniques. Once isolated the different dental stem cells are easily expanded and cryopreserved for long-term storage.
Dental pulp stem cells
The ability of the tooth to provide limited repair by production of new dentine by new odontoblast-like cells, reported by Smith et al.1 and Smith and Lesot,2 suggests that the dental pulp may contain mesenchymal stem cells.
Stem cells from the tooth pulp and several other dental tissues have now been identified and characterised (Fig. 1).3,4,5,6,7,8,9 In 2000 Grontos and collaborators3 first identified a population of cells isolated from the dental pulp of human third molars and termed them dental pulp stem cells (DPSCs). These cells were characterised by their high proliferation and colony-forming properties and when compared to bone marrows cells (BMSCs) in vitro, shared similar immunophenotype. These cells were shown to produce sporadic but densely calcified nodules.3 When transplanted in vivo (using immunocompromised mice as hosts), the cells derived from dental pulp (DPSCs) generated functional dental tissue in the form of dentine/pulp-like complexes.4
Moreover, further characterisation revealed that DPSCs were capable of differentiating into adipoctyes,4 osteoblasts and endoteliocytes.10
This evidence gave indications that DPSCs may have a broader capacity for differentiation than originally expected and this might be due to their developmental origin as neural crest derived cells.
Stem cells from human exfoliated deciduous teeth
Obtaining stem cells from human exfoliated deciduous teeth (SHED) is both simple and convenient.
In 2003 Miura and collaborators identified SHED cells as highly proliferative, clonogenic cells capable of differentiating into a variety of cell types including neural cells, adipocytes and odontoblasts.11 After in vivo transplantation, SHED cells were found to be able to induce bone formation, generate dentine11,12,13 and survive in mouse brain along with expression of neural markers.11 When compared to DPSCs, SHED cells exhibited higher proliferation rates, increased population doublings, osteoinductive capacity in vivo and an ability to form sphere-like clusters.11 Results from in vivo transplantation suggested that SHED have a greater capability for mineralisation than DPSCs.14
Periodontal ligament stem cells
The periodontal ligament (PDL), located between the cementum and the inner wall of the alveolar bone socket, contains specialised connective tissue that provides nourishment to the teeth and regulates periodontal homeostasis. It has long been recognised to contain a population of progenitor cells.15 In 2004, Seo et al.,16 revealed that stem cells from human periodontal ligament (PDLSCs) were capable of differentiating along multilineages to produce cementoblast-like cells and adipocytes. In this study, the PDLSCs extracted from human third molars by single colony selection were characterised as STRO-1/CD146 positive (stem cell markers).16 Moreover, during in vivo studies cementum/PDL-like structures formed, along with dense collagen fibres similar to Sharpey's fibres showing their potential to regenerate PDL attachment, critical in the development and maintenance of a functional tooth.
When the PDL cells were co-transplanted with a different source of dental stem cells derived from the SCAP cells, a construction of root/periodontal complex was formed using a hydroxyapatite/tricalcium phosphate (HA/TCP) carrier into tooth sockets of miniature pigs.17 The root/periodontal complex was found to be capable of supporting a porcelain crown and resulted in normal tooth function.
PDL cells maintain their tissue regeneration capacity even after recovery from frozen human tissue.18 These results suggest the possibility of cryopreserved PDL from extracted teeth being utilised for future therapeutic purposes.
Root apical papilla stem cells
As mentioned in the Introduction, the third molars are the most commonly extracted teeth and very often still not completed their root development, the cells located in the apical papilla (root foramen area) represent another unique population of dental stem cells – SCAP cells.
These cells have the capacity to differentiate into odontoblasts and adipocytes. They have shown a higher proliferative potential compared with DPSCs when measured by bromodeoxyuridine (BrdU) uptake.17
SCAP appear to be a source of primary odontoblasts responsible for the formation of root dentine,17 whereas DPSCs are possibly the source of replacement odontoblasts that produce reparative dentine.3
Dental follicle stem cells
The dental follicle is a loose connective tissue sac surrounding the enamel organ and the dental papilla of the developing tooth germ before eruption.19 Cells isolated from the follicular sacs of human third molars were characterised by their rapid attachment in culture and expression of putative stem cell markers Nestin and Notch-1.20
Their ability to differentiate into cementoblasts was shown in a study using bovine dental follicle cells (BDFCs) that were transplanted in vivo into immunocompromised-SCID mice.21,22
Dental follicle stem cells (DFSCs) can give rise to three major cell types in periodontium: cementoblasts, osteoblasts, and fibroblasts. A recent study from Bay et al. showed that coculture of DFC with Hertwig epithelial root sheath cells in vitro enhances the ability of these cells to regenerate cementum and periodontal ligament after transplantation.23
Gingiva as a source of stem cells
Gingival tissue is one of the important parts of the periodontium, showing remarkable wound healing and regenerative capacity. Gingival fibroblasts are a heterogeneous cell population that play a crucial part in the process of wound healing. They respond differently to growth factors and produce specific extracellular matrix proteins during the healing process.24,25,26,27,28
Progenitor cells and multipotent MSC subpopulation of cells have been isolated and characterised from gingival fibroblasts.29,30,31,32 These fibroblasts are easily accessible and recently have been used to derive induced pluripotent stem cell lines (IPS).33
Oral epithelium
Another very interesting source of stem cells is epithelial cells from the oral epithelium. In 2003 Nakamura and collaborators successfully cultured and transplanted rabbit oral mucosal epithelial cells on amniotic membranes.34 In 2004 transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders was reported.35
A more recent study has shown the expression of angiogenesis-related factors in human corneas after cultivated oral mucosal epithelial transplantation (COMET), suggesting that the expression of FGF-2, VEGF, PEDF, endostatin, and IL-1ra is similar in normal corneas, conjunctiva, oral mucosa, and corneas after COMET.36 A long-term study has shown the results of this technique and suggested it is a very successful way of treatment for severe ocular surface disorders.37
Clinical applications of dental stem cells
Repairing/regenerating dental tissues
The tooth is a complex organ comprising three specialised hard tissues: enamel, dentine and cementum encapsulating the dental pulp and anchored by the periodontal ligament in the alveolar socket of the jaw.
Once damaged, tooth enamel is not capable of self-repairing, while dentine and cementum show limited capacity of regeneration. Studies have revealed that extracellular matrix derivatives and breakdown products from dental pulp and dentine influence pulp cell migration. Recruited cells exhibited increased stem cell marker expression indicating that dental ECMs and their breakdown products selectively attract progenitor cells that contribute to repair processes.38,39
When a tooth is damaged, but still reparable, regeneration of the remaining dental structure can prevent or delay the loss of the whole tooth. When the dental pulp is infected and diagnosed with irreversible pulpitis, regardless of the amount of remaining normal pulp tissue, there is no treatment that can reverse the clinical situation and the entire pulp has to be removed, followed by root canal treatment, disinfecting the pulp space and replacing it with inorganic materials.
The regeneration of the dental pulp and creation of dental pulp that is vital, functionally competent, and able to form and repair dentine has been a long-time quest.
De novo regeneration of dental pulp
The dental pulp has an interesting anatomical location being encased in a mineralised chamber that in many ways resembles bone marrow.
Blood and nerve supply to tooth pulp is, however, restricted by entry through the root tips.
In 2008 Cordeiro and collaborators40 suggested the SHED cells may be a valuable cell source for dental pulp tissue engineering. They seeded SHED cells in biodegradable scaffolds prepared within human tooth slices and transplanted into immunodeficient mice. The resulting tissue presented an architecture and cellularity that resembled those of a physiologic dental pulp. Ultrastructural analysis with transmission electron microscopy and immunohistochemistry for dentine sialoprotein suggested that SHED differentiated into odontoblast-like cells in vivo.40 Huang et al.41 used DPSCs and SCAP cells isolated from human third molars to demonstrate de novo regeneration of dental pulp in empty root canal spaces. These cells were seeded onto poly-D,L-lactide/glycolide scaffold and inserted into the canal space of root fragments followed by subcutaneous transplantation into immunocompromised-SCID mice. After three to four months a histological analysis on the tooth fragments showed that the root canal space was filled with pulp-like tissue with well-established vascularisation. Moreover, a continuous layer of mineralised tissue resembling dentine was deposited on the existing dentinal walls of the canal. This dentine-like structure appeared to be produced by a newly formed layer of odontoblast-like cells.41
Periodontal regeneration
A big challenge in dentistry is the regeneration/repair of the periodontal ligament, particularly since periodontitis is an inflammatory disease with high prevalence, resulting in irreversible loss of connective tissue attachment, supporting alveolar bone and consequent tooth loss. The periodontium is a specialised tissue complex that surrounds and supports the teeth in the alveolar bone.
An area that holds some promise is the use of dental stem cells to replicate the key events in periodontal development both temporally and spatially so that healing can occur in a sequential manner in order to regenerate the periodontium.42
An alternative approach to conventional periodontal regeneration methods was employed by Hasegawa et al.43 involving engineered cell sheets to facilitate human periodontal ligament (HPDL) cell transplantation. Periodontal ligament cells isolated from a human third molar tooth were cultured on poly (N-isopropylacryl-amide) (PIPAAm) dishes that induce spontaneous detachment as viable cell sheets upon low temperature treatment. Athymic rats that had periodontium and cementum removed from their first molars were used to determine the potential of HPDL cell sheets to regenerate periodontal tissues. Fibril anchoring resembling native periodontal ligament fibres together with an acellular cementum-like layer was observed indicating that this technique could be applicable to future periodontal regeneration using dental stem cells.43
In a recent study Tsumanuma et al.44 compared the effects of PDLSC, alveolar periosteum cells, and BMDSCs combined with β-TCP/collagen scaffold transplanted into the bone defect. After eight weeks of transplantation there was a formation of cementum and periodontal ligament fibres. However, the highest alveolar bone regeneration, as well as nerve formation, was observed with PDLSCs.
PDL cells were shown to be capable of forming periodontal tissue around the titanium implants in rats. The potential of these cells to organise the periodontal tissue after tooth loss might open the possibility for new therapeutic approaches in implant therapy and offer an alternative to osseointegrated dental implants.45
In all these approaches, the question remains concerning the extent any reconstituted periodontium can maintain integrity and function over long periods of time, and there is an obvious need for translational and long-term studies. However, current treatments for severe periodontitis are limited and new dental stem cell-based treatments are likely to be the subject of intensive clinical research.
Bone repair
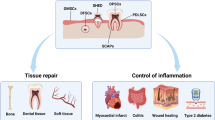
Dental pulp cells are a promising source of MSCs for use in craniomaxillofacial repair and regeneration since they express the osteogenic markers and respond to some growth factors for bone differentiation as osteoblasts. SHED cells transplanted together with hydroxyapatite/tricalcium phosphate into calvarial defects in mice were capable of completely repairing the defect after six months.46
A clinical study revealed that when a collagen sponge scaffold with dental pulp stem/progenitor cells (DPCs) is transplanted into the mandible, oro-maxillofacial (OMF) bone tissue repair with a good vascularisation and lamellar architecture was achieved after one year of the engraftment.47 An important consideration is that dental pulp mesenchymal stem cells are derived from neural crest cells, the same cells from which craniofacial osteoblasts are derived.
Muscle regeneration
The potential of human dental pulp stem cells in muscle regeneration was shown when SHED cells were injected into dogs suffering from muscular dystrophy. Analysis of the DPSCs ability for migration, engraftment, myogenic potential and expression of human dystrophin in affected muscles revealed the formation of chimeric canine/human muscle fibres. A better clinical condition was also observed in the dog, which received monthly arterial injections of SHED cells.48 A recent study done by Martinez and his colleagues49 investigated the potential of PDL cells to support the heart valve cell lineage, specifically the concomitant differentiation to both endothelial cell (EC) and smooth muscle cell (SMC) types, suggesting that PDL cells cultured under steady flow environments can be used in heart valve tissue engineering.49
Immunosuppressive-immunomodulatory properties of dental stem cells
Mesenchymal stem cells have been shown to have immuno-modulatory and regulatory effects on T- and B-lymphocytes, dendritic cells and natural killer cells.50,51,52,53,54,55 These cells do not express MHC class II antigens on their surface when expanded in culture, indicating a possible usage of the mesenchymal stem cells as allogeneic source in treating diseases like graft-versus-host disease (GVHD).56
The dental stem cells are an easily accessible source of mesenchymal stem cells and in 2009 Wada and colleagues showed the immunosuppressive properties of PDL, DP and gingival fibroblast (GF) cells, indicating that these properties might be mediated by soluble factors, produced by activated PBMSC.57 Just one year later, Yamaza et al. showed that SHED cells possess immunomodulatory properties and suggested these cells are an accessible and feasible mesenchymal stem cell source for treating immunodisorders like systematic lupus erythematosus (SLE).58
Whole tooth generation
Functional teeth can be experimentally bioengineered in mice by re-association of dissociated tooth germ cells.59,60,61,62 The re-aggreation produces multiple, small 'toothlets' whose shape bears no resemblance to the shape of the scaffold used (Fig. 2). In this case the role of the scaffold is merely to hold the cells together in a 3D environment. Epithelial and mesenchymal cells are expanded in culture to generate sufficient cells. The two cell populations are combined in vitro bringing the epithelial and mesenchymal cells into direct contact. Interaction between these cell types leads to formation of an early stage tooth primordium. The tooth primordium is surgically transplanted into the mouth and left to develop into a whole bioengineered tooth.
The cells used in these experiments were from embryonic tooth germs and a large number of cells were required to make a tooth. Obviously, this is a problem when trying to translate into the clinic. Thus a major unsolved challenge that we are facing today in the attempts of bioengineering a whole tooth is identifying non-embryonic sources of cells that have tooth-forming abilities following in vitro expansion.
The ability of non-dental cell sources to respond to odontogenic signals following in vitro expansion was demonstrated when it was shown that cultured adult bone marrow stromal cells could form teeth when combined with inductive embryonic oral epithelium.63 In a very recent publication we have shown that epithelial cells derived from adult human gingival tissue are capable of responding to tooth-inducing signals from embryonic mouse tooth mesenchyme, and hybrid teeth with crown and root formation were formed.64
One simple issue that has emerged from using recombination experiments of only human embryonic tooth cells in our lab at King's College is that human bioengineered tooth development follows its own biological clock of development and it takes much longer than when mouse cells are used (unpublished). Thus, whereas development, implantation, growth and eruption of bioengineered mouse teeth might take a few weeks, the equivalent time to create a functional human tooth takes months. Therefore, a future challenge may involve finding a way of accelerating human tooth development.
Concluding remarks
Results to date suggest that teeth are indeed a viable source of adult mesenchymal stem cells for a wide range of clinical applications, despite the obstacles that remain in finding simple and reproducible cell-based approaches for tooth repair and regeneration that could be safely applied in patient treatments.
Teeth are an accessible source of autologous or/and allogeneic mesenchymal stem cells, easily expanded in vitro, maintaining their stemness for long periods and after cryopreservation. Dental stem cells have many advantages over other sources of mesenchymal stem cells and represent a reliable, accessible source of stem cells.
References
Smith A J, Cassidy, N, Perry, H, Beguekirn, C, Ruch, J V, Lesot H . Reactionary Dentinogenesis. Int J Dev Bio 1995; 39: 273–280.
Smith, A J, Lesot H . Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Crit Rev Oral Biol Med 2001; 12: 425–437.
Gronthos, S, Mankani, M, Brahim, J, Robey, P G, Shi S . Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 2000; 97: 13625–13630.
Gronthos S, Brahim J, Li W, Fisher L W et al. Stem cell properties of human dental pulp stem cells. J Dent Res 2002; 81: 531–535.
Jo Y Y, Lee H J, Kook S Y et al. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng 2007; 13: 767–773.
Huang G T, Gronthos S, Shi S . Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 2009; 88: 792–806.
Balic A, Aguila H L, Caimano M J, Francone V P, Mina M . Characterization of stem and progenitor cells in the dental pulp of erupted and unerupted murine molars. Bone 2010; 46: 1639–1651.
Waddington R J, Youde S J, Lee C P, Sloan A J . Isolation of distinct progenitor stem cell populations from dental pulp. Cells Tissues Organs 2009; 189: 268–274.
Koyama N, Okubo Y, Nakao K, Bessho K . Evaluation of pluripotency in human dental pulp cells. J Oral Maxillofac Surg 2009; 67: 501–506.
d'Aquino R, Graziano A, Sampaolesi M et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ 2007; 14: 1162–1171.
Miura M, Gronthos S, Zhao M et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci 2003; 100: 5807–5812.
Shi S, Bartold P M, Miura M, Seo B M, Robey P G, Gronthos S . The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res 2005; 8: 191–199.
Sakai V T, Zhang Z, Dong Z et al. SHED differentiate into functional odontoblasts and endothelium. J Dent Res 2010; 89: 791–796.
Wang X, Sha X J, Li G H et al. Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch Oral Biol 2012; 57: 1231–1240.
McCulloch C A . Progenitor cell populations in the periodontal ligament of mice. Anat Rec 1985; 211: 258–262.
Seo B M, Miura M, Gronthos S et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004; 364: 149–155.
Sonoyama W, Liu Y, Fang D et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 2006; 20: 1: e79.
Seo B M, Miura M, Sonoyama W, Coppe C, Stanyon R, Shi S . Recovery of stem cells from cryopreserved periodontal ligament J Dent Res 2005; 84: 907–912.
Ten Cate A R . Oral histology - development, structure, and function. 5th edn. St Louis, MO: Mosby, 1998.
Morsczeck C, Gotz W, Schierholz J et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol 2005; 24: 155–165.
Handa K, Saito M, Tsunoda A et al. Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect Tissue Res 2002; 43: 406–408.
Handa K, Saito M, Yamauchi M et al. Cementum matrix formation in vivo by cultured dental follicle cells. Bone 2002; 31: 606–611.
Bai Y, Bai K, Matsuzaka S et al. Cementum-and periodontal ligament–like tissue formation by dental follicle cell sheets co-cultured with Hertwig's epithelial root sheath cells. Bone 2011; 48: 1417–1426.
Pitaru S, McCulloch C A, Narayanan S A . Cellular origins and differentiation control mechanisms during periodontal development and wound healing. J Periodontal Res 1994; 29: 81–94.
Sempowski G D, Borrello M A, Blieden T M, Barth R K, Phipps R P . Fibroblast heterogeneity in the healing wound. Wound Repair Regen 1995; 3: 120–131.
Schor S L, Ellis I, Irwin C R et al. Subpopulations of fetal-like gingival fibroblasts: characterisation and potential significance for wound healing and the progression of periodontal disease. Oral Dis 1996; 2: 155–166.
Phipps R P, Borrello M A, Blieden T M . Fibroblast heterogeneity in the periodontium and other tissues. J Periodontal Res 1997; 32: 159–165.
Häkkinen L, Uitto V J, Larjava H . Cell biology of gingival wound healing. Periodontol 2000; 24: 127–152.
Widera D, Zander C, Heidbreder M et al. Adult palatum as a novel source of neural crest-related stem cells. Stem Cells 2009; 27: 1899–1910.
Mitrano T I, Grob M S, Carrión F et al. Culture and characterization of mesenchymal stem cells from human gingival tissue. J Periodontol 2010; 81: 917–925.
Fournier B P, Ferre F C, Couty L et al. Multipotent progenitor cells in gingival connective tissue. Tissue Eng Part A. 2010; 16: 2891–2899.
Hsu S H, Huang G S, Feng F . Isolation of the multipotent MSC subpopulation from human gingival fibroblasts by culturing on chitosan membranes. Biomaterials 2012; 33: 2642–2655.
Wada N, Wang B, Lin N H, Laslett A L, Gronthos S, Bartold P M . Induced pluripotent stem cell lines derived from human gingival fibroblasts and periodontal ligament fibroblasts. J Periodontal Res 2011; 46: 438–447.
Nakamura T, Endo K, Cooper L J et al. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci 2003; 44: 106–116.
Nakamura T, Inatomi T, Sotozono C et al. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol 2004; 88: 1280–1284.
Chen H C, Yeh L K, Tsai Y J et al. Expression of angiogenesis-related factors in human corneas after cultivated oral mucosal epithelial transplantation. Invest Ophthalmol Vis Sci 2012; 53: 5615–5623.
Nakamura T, Takeda K, Inatomi T, Sotozono C, Kinoshita S . Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br J Ophthalmol 2011; 95: 942–946.
Goldberg M, Smith A J . Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med 2004; 15: 13–27.
Smith J G, Smith A J, Shelton R M, Cooper P R . Recruitment of dental pulp cells by dentine and pulp extracellular matrix components. Exp Cell Res 2012; 318: 2397–2406.
Cordeiro M M, Dong Z, Kaneko T et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 2008; 34: 962–969.
Huang G T, Yamaza T, Shea L D et al. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A 2010; 16: 605–615.
Lin N H, Gronthos S, Bartold P M . Stem cells and periodontal regeneration. Aust Dent J 2008; 53: 108–121.
Hasegawa M, Yamato M, Kikuchi A, Okano T, Ishikawa I . Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng 2005; 11: 469–478.
Tsumanuma Y, Iwata T, Washio K et al. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials 2011; 325: 5819–5825.
Lin Y, Gallucci G O, Buser D, Bosshardt D, Belser U C, Yelick P C . Bioengineered periodontal tissue formed on titanium dental implants J Dent Res 2011; 90: 251–256.
Seo B M, Sonoyama W, Yamaza T et al. SHED repair critical-size calvarial defects in mice. Oral Dis 2008; 14: 428–434.
D'Aquino R, De Rosa A, Lanza V et al. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater 2009; 18: 75–83.
Kerkis I, Ambrosio C E, Kerkis A et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: local or systemic? J Transl Med 2008; 6: 35.
Martinez C, Rath S, Van Gulden S et al. Periodontal ligament cells cultured under steady flow environments demonstrate potential for use in heart valve tissue engineering. Tissue Eng Part A 2013; 19: 458–466.
Bartholomew A, Sturgeon C, Siatskas M et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Haematol 2002; 30: 42–48.
Uccelli A, Pistoia V, Moretta L . Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol 2007; 28: 219–226.
Corcione A, Benvenuto F, Ferretti E et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006; 107: 367–372.
Rasmusson I, Le Blanc K, Sundberg B, Ringdén O . Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol 2007; 65: 336–343.
Ramasamy R, Fazekasova H, Lam E W, Soeiro I, Lombardi G, Dazzi F . Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation 2007; 83: 71–76.
Spaggiari G M, Capobianco A, Becchetti S, Mingari M C, Moretta L . Mesenchymalstemcell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006; 107: 1484–1490.
Le Blanc K, Frassoni F, Ball L et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004; 363: 1439–1441.
Wada N, Menicanin D, Shi S, Bartold P M, Gronthos S . Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol 2009; 219: 667–676.
Yamaza T, Kentaro A, Chen C et al. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther 2010; 1: 5.
Yamamoto H, Kim E J, Cho S W, Jung H S . Analysis of tooth formation by reaggregated dental mesenchyme from mouse embryo J Electron Microsc (Tokyo) 2003; 52: 559–566.
Duailibi M T, Duailibi S I, Young C S, Bartlett J D, Vacanti J P, Yelick P C . Bioengineered teeth from cultured rat tooth bud cells J Dent Res 2004; 83: 523–528.
Nakao K, Morita R, Saji Y et al. The development of a bioengineered organ germ method. Nat Methods 2007; 4: 227–230.
Ikeda E, Morita R, Nakao K et al. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci U. S. A. 2009; 106: 13475–13480.
Ohazama A, Modino S A, Miletich I, Sharpe P T . Stem-cell-based tissue engineering of murine teeth J Dent Res 2004; 83: 518–522.
Angelova Volponi A, Kawasaki M, Sharpe P T . Adult human gingival epithelial cells as a source for whole-tooth bioengineering. J Dent Res 2013; 92: 329–334.
Acknowledgements
This research is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. We would like to thank Professor Tara Renton for gaining an ethical approval and sponsorship from the R&D Department as well as recruiting patients and collecting samples for our study. Her help was crucial and essential for our work on human dental stem cells. We would also like to thank Edgar Alasdair for his technical help in our previously published and ongoing studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Volponi, A., Sharpe, P. The tooth – a treasure chest of stem cells. Br Dent J 215, 353–358 (2013). https://doi.org/10.1038/sj.bdj.2013.959
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2013.959
This article is cited by
-
Cav1.2 regulated odontogenic differentiation of NG2+ pericytes during pulp injury
Odontology (2023)
-
Periodontal ligament stem cells as a promising therapeutic target for neural damage
Stem Cell Research & Therapy (2022)
-
Future horizons: embedding the evolving science of regenerative dentistry in a modern, sustainable dental curriculum
British Dental Journal (2022)
-
Agarose-based spheroid culture enhanced stemness and promoted odontogenic differentiation potential of human dental follicle cells in vitro
In Vitro Cellular & Developmental Biology - Animal (2021)
-
Comparative differentiation analysis of distinct oral tissue-derived cells in response to osteogenic stimulation
Clinical Oral Investigations (2019)