Abstract
Recent studies have demonstrated cyclooxygenase 2 (COX-2) overexpression in various human malignancies, especially in breast cancer, where COX-2 turned out to be a predictor of poor survival. To evaluate the relation of COX-2 and Ep-CAM overexpression and its prognostic significance, we performed a retrospective study on 212 breast cancer patients with a median follow-up time of 10.5 years. Overexpression of COX-2 in tumour tissue samples was assessed by immunohistochemistry. COX-2 overexpression was found in 48.6% of the tumour samples and was predictive for poor disease-free and overall survival. Univariate analysis revealed a strong correlation between COX-2 and Ep-CAM overexpression (P=0.009). Concurrent COX-2 and Ep-CAM overexpression was present in 21.7% of tumour specimens and had an additive negative impact on disease-free and overall survival. Determination of both tumour markers should help in guiding new therapeutic strategies in patients with invasive breast cancer.
Similar content being viewed by others
Main
Cyclooxygenase-2 (COX-2) is a prostaglandin synthase that catalyses the synthesis of prostaglandin G2 (PGG2) and PGH2 from arachidonic acid. Recent studies have led to the recognition of the importance of COX-2 in tumorigenesis of different tumour types. It has been shown that COX-2 is involved in tumour angiogenesis (Tsujii et al, 1998; Gately, 2000), in suppression of apoptosis (Sheng et al, 1998) and in the promotion of invasiveness (Tsujii et al, 1997). COX-2 overexpression was found in pancreatic (Molina et al, 1999; Okami et al, 1999; Kokawa et al, 2001), oesophageal (Zimmermann et al, 1999), prostate (Yoshimura et al, 2000), lung (Khuri et al, 2001), head and neck cancers (Chan et al, 1999) and in malignant gliomas (Shono et al, 2001). Tsujii et al reported that COX-2 overexpression in intestinal epithelial cells leads to downregulation of adhesion molecules (i.e. cadherins), resulting in an enhanced tumorigenic potential (Tsujii and DuBois, 1995).
Enhanced COX-2 expression in breast cancer was first indicated by reports of elevated prostaglandin levels in breast carcinomas (Bennett et al, 1977), particularly in patients with metastatic disease (Rolland et al, 1980). A key role of COX-2 for the initiation and progression of breast cancer is suggested by the finding that mere overexpression of COX-2 can be sufficient for inducing mammary gland tumorigenesis in transgenic mice (Liu et al, 2001). Notably, in human breast cancer cell lines, a positive correlation was found between invasiveness, metastatic potential and prostaglandin production (Liu and Rose, 1996). Different groups have described the prognostic significance of COX-2 overexpression in breast cancer (Hwang et al, 1998; Ristimaki et al, 2002; Soslow et al, 2000).
We have recently described the prognostic significance of Ep-CAM overexpression in patients with invasive breast cancer (Gastl et al, 2000). Ep-CAM (also called 17-1A, ESA, EGP40, 323/A3) is a 40-kDa transmembrane glycoprotein expressed on most human epithelial cells (Gottlinger et al, 1986). The Ep-CAM glycoprotein functions as a homotypic intercellular adhesion molecule (Litvinov et al, 1994) and has become a target for antibody-mediated immunotherapy with the murine monoclonal antibody edrecolomab (Riethmuller et al, 1998). So far, no data have been reported on the correlation of COX-2 overexpression with Ep-CAM overexpression in human breast cancer. We therefore examined COX-2 and Ep-CAM overexpression in tumour specimens from 212 patients with invasive breast cancer, and analysed the prognostic value of both tumour markers.
Patients and methods
Patient selection
A total number of 212 patients were included in this retrospective analysis. This patient sample represents one-third of all cases with localised invasive breast cancer who were operated at the Department of Surgery, Innsbruck University Hospital, from 1980 to 1992. In fact, all cases for which paraffin-embedded tissue samples were still retrievable from the local pathology repository and for which clinical follow-up data were available, were included. Only patients without evidence of distant metastasis at the time of primary surgery and with well-documented axillary lymph node status were eligible for this analysis. The median age of the patients was 54.2 years (range, 29–85 years). Patients younger than 50 years were considered premenopausal. Of the women, 112 (52.8%) were node-positive and 100 (47.2%) node-negative. After primary surgery the clinical status was documented by re-evaluating each patient at least once annually at the Department of Surgery. The evaluation procedure included physical examination, mammography, abdominal ultrasound and chest radiography. The median follow-up time was 10.5 years (range, 36–240 months). During this observation period 96 patients relapsed. Of a total of 94 deaths, 84 were due to breast cancer, while 10 patients died without documented disease recurrence.
Histopathology
All tumour samples were formalin-fixed, embedded in paraffin wax and stored at the local pathology repository. Haematoxylin- and eosin-stained slides were prepared from each tumour specimen using routine methods and then examined by light microscopy. Histologic type and tumour grade were assessed by one co-author (PO) in a blinded fashion using standard pathology criteria.
Immunohistochemistry
COX-2 overexpression was determined by immunohistochemistry using the murine monoclonal antibody COX-2 (Cayman, USA). Briefly, 5-μm sections were cut from paraffin-embedded tissue blocks, mounted on adhesive-coated glass slides, deparaffinised and rehydrated. Endogenous peroxidase was inactivated by immersing the slides in 0.3% H2O2 in absolute methanol for 20 min at room temperature. Pretreatment consisted of a 15-min incubation period in a water bath at 90°C. After washing in Tris buffer, slides were incubated for 2 h with the primary antibody (COX-2, Cayman, USA, dilution 1 : 100). Afterwards, a peroxidase-conjugated goat anti-mouse antibody ready-to-use (EnVision™, DAKO, Vienna, Austria) was added for 30 min. For immunostaining, slides were then placed into the chromogen consisting of a diaminobenzidine solution. Finally, slides were counterstained with Mayer's Hemalum solution. In addition, slides were immunostained for Ep-CAM essentially as described previously (Gastl et al, 2000; Spizzo et al, 2002).
Evaluation of slides
COX-2 overexpression was evaluated by two independent assessors (GS and PO) using light microscopy. Reading of tissue slides was blinded, and both assessors were unaware of clinical outcome. COX-2 expression was defined as the presence of specific staining in the cytoplasm of tumour cells. A final expression score was calculated for each tissue sample by multiplying a staining intensity score (0, negative; 1, weak; 2, moderate; 3, strong staining) with a proportion score of positively stained cells (1, 1–10%; 2, 11–50%; 3, 50–80%; 4, 80–100 %). Only samples with a final expression score >4 were defined as ‘overexpressing’. Ep-CAM overexpression was evaluated as previously reported (Gastl et al, 2000; Spizzo et al, 2002).
Statistical methods
Statistical analysis was performed with the SPSS software program for Windows™. The primary end points in this study were disease-free and overall survival. Thus, survival curves were calculated according to the method of Kaplan and Meier. P-values were evaluated using the log-rank test for censored survival data. Follow-up time was censored if the patient was lost to follow-up. Patients who died without documented disease recurrence were considered censored for disease-free survival but were included as deaths for overall survival analysis. The relation between antigen overexpression and other clinical or tumour parameters was calculated with the χ2 test. To determine the relative importance of COX-2 and Ep-CAM overexpression and established prognostic markers, these variables were subjected to multivariate analysis (Cox regression).
Results
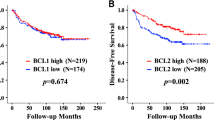
In normal mammary epithelium COX-2 showed absent to weak staining (Figure 1B). COX-2 overexpression in tumour tissue (Figure 1A) was found in 103 of 212 (48.6%) tumour specimens and correlated with poor disease-free (P=0.02, Figure 2A) and overall survival (P=0.04, Figure 2B). Remarkably, COX-2 overexpression was significantly correlated with Ep-CAM overexpression (P<0.009; χ2 test), histologic tumour type (P=0.011) and menopausal status (p=0.047) but failed to correlate with Her-2/neu status or other tumour parameters (Table 1). In 46 (21.7%) of the tumour specimens overexpression of both COX-2 and Ep-CAM was found, while 79 (37.3%) showed neither COX-2 nor Ep-CAM overexpression. Further, three distinct subgroups were identified by the expression of COX-2 and Ep-CAM antigens (Figure 3A, B). Patients with tumours overexpressing both antigens carried the poorest prognosis. Median disease-free and median overall survival time in this patient population were 55 months and 90 months, respectively. Patients overexpressing either COX-2 or Ep-CAM had an intermediate prognosis with a median time to relapse of 127 months and a median survival time of 147 months. Finally, patients without overexpression of COX-2 and Ep-CAM in their tumours carried the best prognosis. Median time to relapse and median survival time for this patient group were not reached. By subgroup analysis, overexpression of COX-2 in node-positive cases predicted a dismal prognosis regarding disease-free and overall survival, whereas in node-negative cases COX-2 overexpression was of no prognostic value (data not shown). By multivariate analysis, nodal status, Ep-CAM overexpression, tumour size and histologic grade, but not COX-2 overexpression, proved to be independent prognostic variables for overall survival. For disease-free survival, nodal status, tumour size and Ep-CAM overexpression, but not COX-2 overexpression, were independent prognostic factors (Table 2).
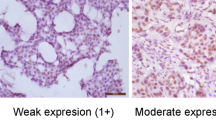
(A) Example of an invasive ductal carcinoma with strong cytoplasmic COX-2 staining, classified as tumour with COX-2 overexpression. (B) Tumour sample showing COX-2 overexpression in invasive lobular carcinoma surrounding normal epithelium lacking COX-2 expression (arrows) as internal negative control. (C) Example of invasive ductal carcinoma presenting with strong membraneous Ep-CAM staining, classified as tumour with Ep-CAM overexpression. (D) Invasive ductal carcinoma without Ep-CAM expression as negative control.
COX-2 overexpression as prognostic marker in a patient sample of 212 breast cancer patients. Patients with tumour tissue presenting COX-2 overexpression (COX-2+) had a significant shortened disease-free intervall (A) and overall survival (B) as compared to patients with tumours lacking COX-2 overexpression (COX-2−).
Relationship between COX-2 and Ep-CAM overexpression with disease-free survival (A) and overall survival (B). COX-2+/Ep-CAM+: patients with tumours overexpressing both antigens, with a median disease-free and overall survival of 55 and 90 months, respectively. COX-2+/−/Ep-CAM+/−: patients with tumours overexpressing only one of the two antigens, with a median disease-free and overall survival of 127 and 147 months, respectively. COX-2−/Ep-CAM−: patients with tumours without overexpression of the antigens, where median disease-free and overall survival were not reached.
Discussion
Our study on 212 patients with invasive breast cancer confirms previous reports that COX-2 overexpression is rather frequent in this patient population (Soslow et al, 2000) and predicts a dismal prognosis for breast cancer patients (Ristimaki et al, 2002).
In experimental studies, COX-2 expression was related to local tumour invasiveness and metastatic potential (Tsujii et al, 1997). It has recently been demonstrated that COX-2 enhances angiogenesis, an effect that can be blocked by selective COX-2 inhibitors (Masferrer et al, 2000). Thus, COX-2 overexpression may provide a clinically useful biomarker for estimating tumour aggressiveness and patients' prognosis.
In our series, COX-2 overexpression was found to be absent in normal mammary gland epithelium surrounding malignant tissue. This observation is in keeping with recent data showing frequently higher COX-2 expression in various epithelial neoplasia compared with adjacent normal tissue (Soslow et al, 2000; Ristimaki et al, 2002).
Ep-CAM expression was found to correlate with cell proliferation and dedifferentiation in epithelial cells (de Boer et al, 1999). To date, little is known about the molecular mechanisms responsible for the regulation of the Ep-CAM gene. The highly significant association of COX-2 and Ep-CAM overexpression suggests a linkage between COX-2 and Ep-CAM signalling. Indeed, Tsujii and DuBois (1995) demonstrated that COX-2 can disrupt cell adhesion mediated by cadherins. Downregulation of cadherins in turn can upregulate Ep-CAM expression. Moreover, cytokines such as IFNα have been shown to upregulate both COX-2 and Ep-CAM expression in epithelial tumour cells (Bostrom et al, 2001; Flieger et al, 2001). Notably, no correlation was found between Her-2/neu and COX-2 overexpression. This finding is somewhat unexpected, since at least in colorectal cells, COX-2 can be upregulated by Her-2/neu receptor signalling (Vadlamudi et al, 1999). Taken together, upon validation in prospective studies, the combination of COX-2 and Ep-CAM expression may significantly improve the estimation of breast cancer prognosis. Beside this, COX-2 and Ep-CAM expression have come into focus as novel targets for therapeutic interventions in colorectal cancer. It remains to be seen whether COX-2 inhibitors and Ep-CAM directed monoclonal antibodies turn out to be efficacious for the treatment of other epithelial cancers such as breast carcinoma.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bennett A, Charlier EM, McDonald AM, Simpson JS, Stamford IF, Zebro T (1977) Prostaglandins and breast cancer. Lancet 2: 624–626
Bostrom PJ, Uotila P, Rajala P, Nurmi M, Huhtaniemi I, Laato M (2001) Interferon-alpha inhibits cyclooxygenase-1 and stimulates cyclo-oxygenase-2 expression in bladder cancer cells in vitro. Urol Res 29: 20–24
Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, Masferrer JL, Dannenberg AJ (1999) Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res 59: 991–994
de Boer CJ, van Krieken JH, Janssen-van Rhijn CM, Litvinov SV (1999) Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. J Pathol 188: 201–206
Flieger D, Hoff AS, Sauerbruch T, Schmidt-Wolf IG (2001) Influence of cytokines, monoclonal antibodies and chemotherapeutic drugs on epithelial cell adhesion molecule (EpCAM) and LewisY antigen expression. Clin Exp Immunol 123: 9–14
Gastl G, Spizzo G, Obrist P, Dunser M, Mikuz G (2000) Ep-CAM overexpression in breast cancer as a predictor of survival. Lancet 356: 1981–1982
Gately S (2000) The contributions of cyclooxygenase-2 to tumor angiogenesis. Cancer Metast Rev 19: 19–27
Gottlinger HG, Funke I, Johnson JP, Gokel JM, Riethmuller G (1986) The epithelial cell surface antigen 17-1A, a target for antibody-mediated tumor therapy: its biochemical nature, tissue distribution and recognition by different monoclonal antibodies. Int J Cancer 38: 47–53
Hwang D, Scollard D, Byrne J, Levine E (1998) Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst 90: 455–460
Khuri FR, Wu H, Lee JJ, Kemp BL, Lotan R, Lippman SM, Feng L, Hong WK, Xu XC (2001) Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res 7: 861–867
Kokawa A, Kondo H, Gotoda T, Ono H, Saito D, Nakadaira S, Kosuge T, Yoshida S (2001) Increased expression of cyclooxygenase-2 in human pancreatic neoplasms and potential for chemoprevention by cyclooxygenase inhibitors. Cancer 91: 333–338
Litvinov SV, Velders MP, Bakker HA, Fleuren GJ, Warnaar SO (1994) Ep-CAM: a human epithelial antigen is a homophilic cell–cell adhesion molecule. J Cell Biol 125: 437–446
Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T (2001) Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem 276: 18563–18569
Liu XH, Rose DP (1996) Differential expression and regulation of cyclooxygenase-1 and -2 in two human breast cancer cell lines. Cancer Res 56: 5125–5127
Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K (2000) Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res 60: 1306–1311
Molina MA, Sitja-Arnau M, Lemoine MG, Frazier ML, Sinicrope FA (1999) Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines: growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Res 59: 4356–4362
Okami J, Yamamoto H, Fujiwara Y, Tsujie M, Kondo M, Noura S, Oshima S, Nagano H, Dono K, Umeshita K, Ishikawa O, Sakon M, Matsuura N, Nakamori S, Monden M (1999) Overexpression of cyclooxygenase-2 in carcinoma of the pancreas. Clin Cancer Res 5: 2018–2024
Riethmuller G, Holz E, Schlimok G, Schmiegel W, Raab R, Hoffken K, Gruber R, Funke I, Pichlmaier H, Hirche H, Buggisch P, Witte J, Pichlmayr R (1998) Monoclonal antibody therapy for resected Dukes' C colorectal cancer: seven-year outcome of a multicenter randomized trial. J Clin Oncol 16: 1788–1794
Ristimaki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J (2002) Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res 62: 632–635
Rolland PH, Martin PM, Jacquemier J, Rolland AM, Toga M (1980) Prostaglandin in human breast cancer: evidence suggesting that an elevated prostaglandin production is a marker of high metastatic potential for neoplastic cells. J Natl Cancer Inst 64: 1061–1070
Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN (1998) Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res 58: 362–366
Shono T, Tofilon PJ, Bruner JM, Owolabi O, Lang FF (2001) Cyclooxygenase-2 expression in human gliomas: prognostic significance and molecular correlations. Cancer Res 61: 4375–4381
Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT (2000) COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 89: 2637–2645
Spizzo G, Obrist P, Ensinger C, Theurl I, Dunser M, Ramoni A, Gunsilius E, Eibl G, Mikuz G, Gastl G (2002) Prognostic significance of Ep-CAM AND Her-2/neu overexpression in invasive breast cancer. Int J Cancer 98: 883–888
Tsujii M, DuBois RN (1995) Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 83: 493–501
Tsujii M, Kawano S, DuBois RN (1997) Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA 94: 3336–3340
Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN (1998) Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93: 705–716
Vadlamudi R, Mandal M, Adam L, Steinbach G, Mendelsohn J, Kumar R (1999) Regulation of cyclooxygenase-2 pathway by HER2 receptor. Oncogene 18: 305–314
Yoshimura R, Sano H, Masuda C, Kawamura M, Tsubouchi Y, Chargui J, Yoshimura N, Hla T, Wada S (2000) Expression of cyclooxygenase-2 in prostate carcinoma. Cancer 89: 589–596
Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schror K (1999) Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res 59: 198–204
Acknowledgements
We thank Ms Ines Tschörner (Institute of Pathology) for her excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Spizzo, G., Gastl, G., Wolf, D. et al. Correlation of COX-2 and Ep-CAM overexpression in human invasive breast cancer and its impact on survival. Br J Cancer 88, 574–578 (2003). https://doi.org/10.1038/sj.bjc.6600741
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6600741
Keywords
This article is cited by
-
S-nitrosylated and non-nitrosylated COX2 have differential expression and distinct subcellular localization in normal and breast cancer tissue
npj Breast Cancer (2020)
-
Expression and function of epithelial cell adhesion molecule EpCAM: where are we after 40 years?
Cancer and Metastasis Reviews (2020)
-
Cross-talk between SIM2s and NFκB regulates cyclooxygenase 2 expression in breast cancer
Breast Cancer Research (2019)
-
Euterpe oleracea extract inhibits tumorigenesis effect of the chemical carcinogen DMBA in breast experimental cancer
BMC Complementary and Alternative Medicine (2018)
-
Increased COX-2 expression in epithelial and stromal cells of high mammographic density tissues and in a xenograft model of mammographic density
Breast Cancer Research and Treatment (2015)