Abstract
Hypoxia-inducible factor-1 (HIF-1) plays important roles in regulating radiosensitivity, making it a potentially promising target for tumour radiosensitisation. Here, we discuss the rationale for, and the potential pitfalls of, combining HIF-1 blockade with radiotherapy. In doing so, we describe clinical scenarios in which HIF-1 inhibition might optimise tumour radiosensitivity.
Similar content being viewed by others
Main
Hypoxia is an important contributor to tumour radioresistance (Brizel et al, 1997). Oxygen increases the cytotoxicity of radiation, resulting in roughly a three-fold difference in radiosensitivity between hypoxic and aerobic cells. This phenomenon, termed the oxygen effect, is widely attributed to oxygen's ability to chemically modify radiation-induced DNA damage, creating adducts that are not easily repaired by cells (Roots and Smith, 1974).
As we have come to better understand how tumours respond and adapt to hypoxia, it has become apparent that there may also be biological mechanisms that contribute to the oxygen effect. Owing to an imbalance in oxygen supply and demand, most solid tumours are hypoxic (Dewhirst, 2003). As a result, signaling pathways stimulated by hypoxia are commonly activated in tumours (Harris, 2002). Arguably, the best understood of these pathways is controlled by hypoxia-inducible factor-1 (HIF-1). Hypoxia-inducible factor-1 is a transcription factor activated by hypoxia that modulates more than 100 genes involved in regulating important processes such as metabolism, proliferation, apoptosis, and angiogenesis (Semenza, 2003).
Teleologically, the downstream effects of HIF-1 serve to help the cell adapt to hypoxic stress. In doing so, they change the tumour phenotype in ways that might also impact radiosensitivity; some positively, and some negatively (Table 1). As some of these HIF-1-mediated processes will be more predominant in certain tumours, it is likely that HIF-1 may have varying effects on radiosensitivity from tumour to tumour. In fact, there are some clinical data, which support this concept. In oropharyngeal cancer, high levels of HIF-1 expression have been shown to predict for poor local control in advanced disease (Aebersold et al, 2001), while predicting for superior local control in early-stage disease (Fillies et al, 2005).
The above data suggest that HIF-1 influences tumour radiosensitivity, but that the degree and direction of that influence may be dependent on context. Here, we hope to begin building an understanding for how the tumour phenotype affects the relationship between HIF-1 and tumour radiosensitivity. We will attempt to extrapolate from this how HIF-1 blockade might best be used to capitalise on its potential for tumour radiosensitisation.
How radiation affects HIF-1
As shown by the clinical data mentioned above, pretreatment HIF-1 expression levels influence local control for irradiated tumours. This raises the question of how HIF-1 activity varies during and after radiotherapy, as these are the times when the protein should function as a modulator of radioresistance. As it turns out, radiation causes HIF-1 expression levels to increase in tumours (Moeller et al, 2004). The effect is dose-responsive, but does not appear to depend on dose fractionation. The upregulation begins as early as 24 h after treatment, and has been shown to endure for as long as 1 week. Coincident with this effect, several important downstream targets of HIF-1 are also upregulated in irradiated tumours. These proteins, including vascular endothelial growth factor (VEGF), plasminogen activator inhibitor-1, and carbonic anhydrase IX, serve as the longer-lived mediators of the radiation-induced HIF-1 effect.
The mechanisms behind this effect are interesting and, importantly, reveal nuances that may be clinically relevant. Two pathways have been worked out to explain how irradiation leads to HIF-1 activation (Moeller et al, 2004). The two share a common origin: radiation-induced tumour reoxygenation. As mentioned above, radiation preferentially kills well-oxygenated and highly metabolic tumour cells. The death of these cells frees up oxygen to distribute to regions of the irradiated tumour that were previously hypoxic. Tumours are also debulked by the cell death occurring after radiation, freeing up space for vessels to expand and improve blood flow and nutrient delivery to starved regions of tumour tissue. The end result is that oxygen levels in tumour tissue tend to be higher after irradiation, an effect termed ‘reoxygenation’.
Paradoxically, increasing tumour oxygenation in this way causes activation of the HIF-1 pathway, which is normally responsive to decreased oxygenation. Part of the explanation for this comes from the oxidative stress generated during tumour reoxygenation. After irradiation, during reoxygenation, free radical species accumulate in tumour tissue and lead to overexpression of HIF-1 (Moeller et al, 2004). It is unknown precisely through what mechanism this occurs, but several theories have been put forward. One postulates that free radical species generated in the mitochondria during hypoxia are the signal used by the cell to sense oxygen deprivation and that they are, therefore, capable themselves of mimicking hypoxia (Chandel et al, 2000). Another suggests that reactive nitrogen species inhibit the cellular machinery that breaks down HIF-1 during normoxia, causing HIF-1 to function as though the cells were hypoxic (Metzen et al, 2003). But whatever the cause, reoxygenation clearly changes the redox environment in irradiated tumours, causing accumulation of HIF-1.
Apart from setting off this oxidative pathway, reoxygenation also affects the translational machinery in the irradiated tumour cell, further contributing to the radiation-induced activation of the HIF-1 pathway. When a cell becomes stressed, it strives to conserve energy by, in part, slowing down protein synthesis. This is accomplished through a variety of mechanisms (Wouters et al, 2005). One involves dynamic regulation of protein translation through so-called stress granules. Stress granules are cytosolic polymers made up of mRNA, ribosomal subunits, and various other proteins (Kedersha et al, 1999). They serve as points of triage during stress, differentiating which transcripts need urgent translation and which can be sequestered in the granule for translation at a more favourable point in time (Kedersha and Anderson, 2002). As long as the cell has not been irreversibly damaged, the granules depolymerise once the stress is alleviated, and the once-sequestered transcripts go on to be translated normally.
Stress granules play a role in activating the HIF-1 pathway after tumours are irradiated (Moeller et al, 2004). They form in response to hypoxia and bind, among other things, mRNA transcribed off of HIF-1-regulated genes. This effect is not absolute, as proteins downstream of HIF-1 are synthesised during hypoxia. However, the magnitude of their upregulation is blunted by the activity of the stress granules. As proof of this, when reoxygenation causes the stress granules to disassemble, new protein translation causes synthesis of downstream HIF-1 targets to increase approximately two-fold. This provides the second mechanism through which radiation upregulates HIF-1 activity in tumours.
In summary, radiation causes upregulation of the HIF-1 pathway in tumours through two mechanisms dependent on reoxygenation. The resulting increased levels of free radical species within the tumour caused the HIF-1 protein to accumulate. At the same time, stress granules are depolymerised, reversing a partial blockade on protein synthesis in the HIF-1 pathway. Together, these effects significantly upregulate the HIF-1 pathway in the irradiated tumour.
How HIF-1 affects tumour radiation response
As discussed above, data exists to suggest that HIF-1 plays a role in determining tumour radiosensitivity. Until recently, no study had been carried out to examine the mechanisms behind this relationship. These details are important to understand as they may lend further insight into why HIF-1 may differentially radiosensitise certain tumours. Moreover, this knowledge may reveal which tumours might be maximally radiosensitised by HIF-1 blockade.
To date, the effect HIF-1 has on tumour radiosensitivity has been ascribed to four different processes: its impact on apoptosis, metabolism, proliferation, and the tumour vasculature. It is not yet known whether any of these contributes more or less than the others to the overall effect so, for now, each should be considered as important as the next. We will examine each, in turn, below.
In general, HIF-1 has a complicated relationship with apoptosis. As mentioned above, HIF-1 has been shown in different situations to be both proapoptotic and antiapoptotic. In the irradiated cell, however, HIF-1 appears to have a net proapoptotic effect. Normally, ionising radiation induces breaks in DNA that are sensed by the cell, setting off a cascade of molecular reactions that help determine which of the many possible fates (i.e. repair, apoptosis, mitotic death) the cell should meet. For cells that eventually undergo apoptosis, one of the most important events in this cascade is the activation of p53.
HIF-1 exerts its effect on radiation-induced apoptosis, at least in part, through interacting with p53. Tumour cells exposed to both hypoxia and radiation undergo apoptosis through a p53- and HIF-1-dependent mechanism (Moeller et al, 2005b). HIF-1 enhances phosphorylation of p53 in the irradiated cell, promoting caspase cleavage and, eventually, apoptosis. Of note, HIF-1 appears to have no impact on radiation-induced apoptosis in the p53-null human prostate cell line, PC-3. When p53 is reintroduced to the PC-3 line, it regains HIF-1-dependent sensitivity to radiation-induced apoptosis. It remains to be seen whether the link between HIF-1 and radiation-induced apoptosis is as closely tied to p53 in other cell lines. There is ample reason to believe it may not be, as HIF-1 is known to affect several p53-independent apoptotic mechanisms, such as BNIP3 (Bruick, 2000).
HIF-1 can also affect tumour cell clonogenicity following irradiation by altering cellular metabolism. It governs the expression of a host of proteins involved in glycolysis, and serves an important role in maintaining energy levels during hypoxia. Consequently, tumours deficient in HIF-1 have difficulty maintaining ATP levels (Griffiths et al, 2002; Moeller et al, 2005b) – a state which might increase clonogenicity after irradiation (Luk and Sutherland, 1987). Indeed, if tumour cells are made to be hypoxic in a glucose-limiting environment, without the aid of HIF-1's glycolysis-promoting effects, their metabolic rates drop, ATP levels fall, and in vitro clonogenicity increases (Moeller et al, 2005b).
HIF-1 also affects tumour cell clonogenicity by altering the kinetics of cellular proliferation. This effect is highly dependent on the local microenvironment and, therefore, varies considerably from place to place within a tumour. In regions where both hypoxia and glucose are limited, HIF-1 maintains tumour proliferation, likely by supporting bioenergetics. In regions where hypoxia alone is limiting, HIF-1 promotes cell cycle blockade, likely by modulating p21 and p27 (Goda et al, 2003). The overall impact of these combined effects is that HIF-1 radiosensitises tumours by keeping cells proliferative in nutrient-depleted regions of the tumour tissue (Moeller et al, 2005b).
The final known mechanism linking HIF-1 activity to tumour radiosensitivity involves the regulation of vascular radiosensitivity. The degree to which the tumour vasculature is damaged by radiation has been shown to be an important determinant of overall responsiveness of tumours to ionising radiation in animal models (Garcia-Barros et al, 2003). The radiosensitivity of endothelial cells within a tumour, in turn, appears to be highly dependent on their exposure to proangiogenic stimulants. Proangiogenic cytokines, such as VEGF and basic fibroblast growth factor, have been found to induce significant radioprotection in endothelial cells (Gorski et al, 1999; Paris et al, 2001).
HIF-1, which promotes the expression of a variety of proangiogenic cytokines, is poised to be a major regulator of this behavior in tumours. Conditioned media taken from wild-type tumour cells is much more efficient at inducing resistance to radiation-induced death in endothelial cells than is that taken from HIF-1-deficient tumour cells (Moeller et al, 2004). In line with this finding, the vasculature of HIF-1-deficient tumours undergoes significantly more regression following irradiation than does the vasculature of their wild-type controls (Moeller et al, 2005b). Through its protective effects on the surrounding vascular endothelium, then, HIF-1 serves as a powerful radioprotective factor for tumours.
The above data show that HIF-1 has divergent effects on radiosensitivity: sensitisation of tumour cells and protection of stromal endothelial cells (Figure 1). It is critical to understand how these divergent mechanisms come together to bring about a change in tumour radiosensitivity. If the net result of HIF-1 activity is to promote radioresistance in tumours, HIF-1 blockade would seem to be a promising strategy for tumour radiosensitisation. However, three of the four HIF-1-mediated effects described – enhancing apoptosis, metabolism, and proliferation – result in tumour radiosensitisation, while only one – vascular protection – promotes tumour radioresistance. One might predict from this, then, that HIF-1 blockade could interfere with radiotherapy. Studies have shown, however, that HIF-1-deficient tumours are more radiosensitive than their wild-type counterparts (Zhang et al, 2004; Moeller et al, 2005b; Williams et al, 2005).
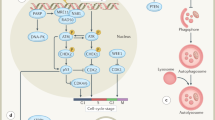
A representation of HIF-1's effects on radiosensitivity for a HIF-1-expressing tumour cell (blue), a non-HIF-1-expressing tumour cell (green), and a tumour vessel (red). Dashed arrows depict the normal effects of ionising radiation. Dotted arrows show HIF-1-mediated pathways that sensitise tumours to radiation. Solid arrows show HIF-1-mediated pathways that protect tumours from radiation, whose blockade might radiosensitise tumours. ‘VEGF’ symbolises the proangiogenic pathway, ‘p21’ the mitotic pathway, ‘p-p53’ the apoptotic pathway, and ‘ATP’ the metabolic pathway. Note that in this schematic, non-HIF-1-expressing cells are still radiosensitised by HIF-1 blockade.
How can this apparent discrepancy be explained? One possibility is that HIF-1 promotes tumour radioresistance through some other effects, not yet realised or described. Another is that the vascular radiosensitisation caused by HIF-1 blockade is a more powerful influence on tumour radiosensitivity than are the other factors discussed. There are some data to support these hypotheses. It has been shown in one experimental system that eliminating the hypoxic fraction of a HIF-1-deficient tumour immediately prior to irradiation does not alter the tumour regrowth rate after treatment (Williams et al, 2005). What this experiment suggests is that, in contrast to wild-type tumours, the hypoxic fraction of HIF-1-deficient tumours does not contribute to tumour regrowth after irradiation. One might surmise from this that even though HIF-1 blockade causes some degree of radioresistance in the hypoxic cell, the combination of hypoxia and HIF-1 inhibition render that cell, overall, nonclonogenic after irradiation. Moreover, since the radiosensitising effects of HIF-1 blockade occur at a distance – damaging all tumour cells fed by the affected vasculature – the positive effects apply more broadly over the tumour than do the negative effects. Whatever the explanation, these data suggest that HIF-1 blockade is a viable strategy for tumour radiosensitisation.
Clinical correlation
There are lessons to be learned from the mechanisms connecting radiation to HIF-1, and HIF-1 to radiosensitivity, which may be relevant for the use of HIF-1 blockade as a tumour radiosensitiser (Table 2). First, the degree of tumour hypoxia is likely to play a role in determining how HIF-1 blockade influences tumour radiosensitivity. Of course, there are numerous hypoxia-independent stimuli for HIF-1 activation (Dery et al, 2005). As a result, tumours that are well-oxygenated yet signal robustly through PI3K, for example, may have sufficient HIF-1 activity to influence their radiosensitivity. However, tumours need to be hypoxic to undergo radiation-induced HIF-1 activation since reoxygenation is required to initiate this pathway. Therefore, both baseline HIF-1 expression levels and oxygenation are likely important determinants of how tumour radiosensitivity will respond to HIF-1 blockade. Second, since stress granules do not depolymerise after a cell experiences a lethal threat, tumours need to be made up of mostly viable tissue in order to maximally activate the HIF-1 pathway after radiation. Therefore, tumours with low HIF-1 activity levels, little or no hypoxia, or tumours with vast amounts of necrosis are unlikely to undergo much HIF-1 activation in response to radiotherapy. Consequently, HIF-1 blockade is less likely to add benefit to radiation in such tumours. Third, since HIF-1 collaborates with p53 to promote apoptosis, scheduling HIF-1 inhibition with radiation may be a challenge in p53-positive tumours.
Considerations such as these may influence how HIF-1 blockade is used in the clinic. This is particularly relevant now, as there are several agents currently being developed as potential HIF-1 inhibitors for use in oncology. One frontrunner is topotecan, a topoisomerase I inhibitor which blocks HIF-1 translation at doses lower than those required for the drug to damage DNA (Rapisarda et al, 2004). Topotecan is currently undergoing a phase one clinical study as a HIF-1 inhibitor. A second promising class of HIF-1-inhibiting agents undergoing active clinical development are the analogues of 2-methoxyestradiol from EntreMed, Inc, which downregulate HIF-1 at the post-translational level (Mabjeesh et al, 2003). A large number of other ‘hits’ from various small molecule screens have also been presented as potential clinical HIF-1 inhibitors. Only time will tell which of these, if any, are efficacious in human tumours and augment the effects of standard cytotoxic therapies such as radiation.
Another potential strategy to counter the radioprotective effects of HIF-1 would be to inhibit the mechanisms by which radiation causes HIF-1 activation. This has been done in a preclinical model using a manganese porphyrin compound – a mimetic of superoxide dismutase – to scavenge the free radical species generated during radiation-induced reoxygenation (Moeller et al, 2004). Combining these agents with radiotherapy delays tumour regrowth significantly over that seen with either treatment alone (Moeller et al, 2005a). As the manganese porphyrin compounds also protect normal tissues from radiation damage, this may be a powerful clinical therapeutic option.
Finally, it is worth mentioning here that there are no data yet on whether HIF-1 blockade might impact normal tissue radiosensitivity. As HIF-1 is not typically active in most healthy normal tissues, one would expect that its inhibition would not affect normal tissue radiosensitivity. There may, however, be exceptions to this rule. There are some normal tissues, such as the liver and the thymus, that are hypoxic at baseline (Arteel et al, 1995; Hale et al, 2002). HIF-1 may play a homeostatic role in such organs, potentially important in the recovery from radiation damage. Indeed, HIF-1 has been implicated in the response to injury and inflammation in normal tissues (Maeno et al, 2005), processes which have much in common with the response to radiation damage. It remains an important yet unresolved issue to determine whether inhibiting the function of HIF-1 in normal tissues would impair their ability to heal after being irradiated.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Aebersold DM, Burri P, Beer KT, Laissue J, Djonov V, Greiner RH, Semenza GL (2001) Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res 61: 2911–2916
Arteel GE, Thurman RG, Yates JM, Raleigh JA (1995) Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer 72: 889–895
Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW (1997) Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 38: 285–289
Bruick RK (2000) Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci USA 97: 9082–9087
Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E (1998) Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394: 485–490
Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT (2000) Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 275: 25130–25138
Dery MA, Michaud MD, Richard DE (2005) Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol 37: 535–540
Dewhirst MW (2003) Mechanisms underlying hypoxia development in tumors. Adv Exp Med Biol 510: 51–56
Fillies T, Werkmeister R, van Diest PJ, Brandt B, Joos U, Buerger H (2005) HIF1-alpha overexpression indicates a good prognosis in early stage squamous cell carcinomas of the oral floor. BMC Cancer 5: 84
Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R (2003) Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 300: 1155–1159
Goda N, Ryan HE, Khadivi B, McNulty W, Rickert RC, Johnson RS (2003) Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol Cell Biol 23: 359–369
Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, Weichselbaum RR (1999) Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res 59: 3374–3378
Griffiths JR, McSheehy PM, Robinson SP, Troy H, Chung YL, Leek RD, Williams KJ, Stratford IJ, Harris AL, Stubbs M (2002) Metabolic changes detected by in vivo magnetic resonance studies of HEPA-1 wild-type tumors and tumors deficient in hypoxia-inducible factor-1beta (HIF-1beta): evidence of an anabolic role for the HIF-1 pathway. Cancer Res 62: 688–695
Hale LP, Braun RD, Gwinn WM, Greer PK, Dewhirst MW (2002) Hypoxia in the thymus: role of oxygen tension in thymocyte survival. Am J Physiol Heart Circ Physiol 282: H1467–H1477
Harris AL (2002) Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer 2: 38–47
Kedersha N, Anderson P (2002) Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans 30: 963–969
Kedersha NL, Gupta M, Li W, Miller I, Anderson P (1999) RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol 147: 1431–1442
Luk CK, Sutherland RM (1987) Nutrient modification of proliferation and radiation response in EMT6/Ro spheroids. Int J Radiat Oncol Biol Phys 13: 885–895
Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P (2003) 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 3: 363–375
Maeno H, Ono T, Dhar DK, Sato T, Yamanoi A, Nagasue N (2005) Expression of hypoxia inducible factor-1alpha during liver regeneration induced by partial hepatectomy in rats. Liver Int 25: 1002–1009
Metzen E, Zhou J, Jelkmann W, Fandrey J, Brune B (2003) Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell 14: 3470–3481
Moeller BJ, Batinic-Haberle I, Spasojevic I, Rabbani ZN, Anscher MS, Vujaskovic Z, Dewhirst MW (2005a) A manganese porphyrin superoxide dismutase mimetic enhances tumor radioresponsiveness. Int J Radiat Oncol Biol Phys 63: 545–552
Moeller BJ, Cao Y, Li CY, Dewhirst MW (2004) Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 5: 429–441
Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY, Dewhirst MW (2005b) Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell 8: 99–110
Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R (2001) Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293: 293–297
Piret JP, Lecocq C, Toffoli S, Ninane N, Raes M, Michiels C (2004) Hypoxia and CoCl2 protect HepG2 cells against serum deprivation- and t-BHP-induced apoptosis: a possible anti-apoptotic role for HIF-1. Exp Cell Res 295: 340–349
Rapisarda A, Uranchimeg B, Sordet O, Pommier Y, Shoemaker RH, Melillo G (2004) Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res 64: 1475–1482
Roots R, Smith KC (1974) On the nature of the oxygen effect on x-ray-induced DNA single-strand breaks in mammalian cells. Int J Radiat Biol Relat Stud Phys Chem Med 26: 467–480
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3: 721–732
Semenza GL, Roth PH, Fang HM, Wang GL (1994) Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem 269: 23757–23763
Williams KJ, Telfer BA, Xenaki D, Sheridan MR, Desbaillets I, Peters HJ, Honess D, Harris AL, Dachs GU, van der Kogel A, Stratford IJ (2005) Enhanced response to radiotherapy in tumours deficient in the function of hypoxia-inducible factor-1. Radiother Oncol 75: 89–98
Wouters BG, van den Beucken T, Magagnin MG, Koritzinsky M, Fels D, Koumenis C (2005) Control of the hypoxic response through regulation of mRNA translation. Semin Cell Dev Biol 16: 487–501
Zhang X, Kon T, Wang H, Li F, Huang Q, Rabbani ZN, Kirkpatrick JP, Vujaskovic Z, Dewhirst MW, Li CY (2004) Enhancement of hypoxia-induced tumor cell death in vitro and radiation therapy in vivo by use of small interfering RNA targeted to hypoxia-inducible factor-1alpha. Cancer Res 64: 8139–8142
Acknowledgements
This work was supported by the Duke SPORE for breast cancer, NIH grant CA40355, the Howard Hughes Medical Institute, and the Duke Medical Scientist Training Program grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Moeller, B., Dewhirst, M. HIF-1 and tumour radiosensitivity. Br J Cancer 95, 1–5 (2006). https://doi.org/10.1038/sj.bjc.6603201
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6603201
Keywords
This article is cited by
-
Interfering with Tumor Hypoxia for Radiotherapy Optimization
Journal of Experimental & Clinical Cancer Research (2021)
-
Transportable system enabling multiple irradiation studies under simultaneous hypoxia in vitro
Radiation Oncology (2018)
-
Overexpression of HOTAIR leads to radioresistance of human cervical cancer via promoting HIF-1α expression
Radiation Oncology (2018)
-
SIM2l attenuates resistance to hypoxia and tumor growth by transcriptional suppression of HIF1A in uterine cervical squamous cell carcinoma
Scientific Reports (2017)
-
Inhibition of Notch and HIF enhances the antitumor effect of radiation in Notch expressing lung cancer
International Journal of Clinical Oncology (2017)