Abstract
Background:
Protein tyrosine kinase 6 (PTK6; breast tumour kinase) is overexpressed in up to 86% of the invasive breast cancers, and its association with the oncoprotein human epidermal growth factor receptor 2 (HER2) was shown in vitro by co-precipitation. Furthermore, expression of PTK6 in tumours is linked with the expression of HER2.
Method and results:
In this study, we used the proximity ligation assay (PLA) technique on formalin-fixed paraffin sections from eighty invasive breast carcinoma tissue specimens to locate PTK6–HER2 protein–protein complexes. Proximity ligation assay signals from protein complexes were assessed quantitatively, and expression levels showed a statistically significant association with tumour size (P=0.015) and course of the cancer disease (P=0.012).
Conclusion:
Protein tyrosine kinase 6 forms protein complexes with HER2 in primary breast cancer tissues, which can be visualised by use of the PLA technique. Human epidermal growth factor receptor 2–PTK6 complexes are of prognostic relevance.
Similar content being viewed by others
Main
Although cytoplasmic protein tyrosine kinase 6 (PTK6; breast tumour kinase (BRK)) is overexpressed in up to 86% of breast carcinomas (Ostrander et al, 2007; Harvey et al, 2009; Brauer and Tyner, 2010), its physiological role is rather unclear. However, various in vitro studies have suggested its possible involvement in modulating signal transduction of human epidermal growth factor receptor (HER) tyrosine kinases (Haegebarth et al, 2005; Zhang et al, 2005; Ostrander et al, 2007; Aubele et al, 2008), as well as influencing transcriptional activities (Liu et al, 2006; Weaver and Silva, 2007) and proliferation (Harvey and Crompton, 2003). Using immunohistochemistry on breast cancer tissues, PTK6 expression was shown to be associated with the expression of HER receptors (Aubele et al, 2007, 2008; Xiang et al, 2008), and with the course of the breast cancer disease (Aubele et al, 2007).
In this study, we applied the proximity ligation assay (PLA) technique (Söderberg et al, 2006), to examine the presence of PTK6–HER2 protein–protein interactions in 80 primary invasive breast carcinoma tissue specimens. As a result, we give evidence that PTK6–HER2 protein complex formation is associated with tumour size and patient outcome.
Materials and methods
Transfection of T47D cells
A total of 106 T47D cells growing in antibiotic-free RPMI medium (Aubele et al, 2008) were transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) and siRNAs (each 200 pmol, Thermo Scientific, Dharmacon, Chicago, IL, USA) targeting PTK6 and HER2, respectively, according to the manufacturers’ instructions. As controls, we added transfection reagents without siRNAs to the control cells (mock transfection), and performed transfection of the control cells with siRNA targeting GAPDH. After 24 h, one part of the cells was further cultivated in complete medium for 48 h and then used for protein isolation and western blot analysis. The other part of cells was placed on microscopic slides (2.5 × 105 cells per slide; SuperFrost, Carl Roth, Karlsruhe, Germany) and further cultivated for 48 h. Subsequently, the slides were washed in PBS and fixed with 4% paraformaldehyde for 15 min at 22°C.
Western blot analysis
T47D cells were washed in PBS, sedimented, and lysed as described previously (Aubele et al, 2008). Then, PTK6, HER2, and GAPDH protein expressions were detected by primary antibodies against PTK6 (sc-1188, Santa Cruz, Biotech, Heidelberg, Germany), GAPDH (sc-25778, Santa Cruz Biotech, Heidelberg, Germany), HER2 (A0485, DAKO, Glostrup, Denmark), and tubulin (T5168, Sigma, St Louis, MO, USA) as loading control. Peroxidase-conjugated secondary antibodies were added (NA934V and NA931V; GE Healthcare, Chalfont St Giles, Buckinghamshire, UK). Band intensities were quantified densitometrically relative to the appropriate control bands using the Molecular Imager ChemiDoc XRS and the software Quantity One (Bio-Rad Laboratories, Hercules, CA, USA).
Patients and tumour samples
Tumour samples from 80 patients were randomly selected, and all tumour patients were surgically treated. None of the patients received any preoperative treatment, and there was no correlation between the adjuvant treatment regimes and our results. The median time of follow-up of patients was 139 months (maximum 252 months) with 24 (30%) of the patients experiencing distant disease recurrences. Further information of the samples is summarised in Table 1. For all of the patients, the immunohistochemical data for HER2 status (Hercep test, K5204, DAKO, Hamburg, Germany) and for PTK6 expression (BRK, sc-1188, Santa Cruz, Biotech, Heidelberg, Germany) were available as published elsewhere (Aubele et al, 2008).
Proximity ligation assay technique
From tissue microarray (TMA), blocks containing primary breast cancer tissues of (Aubele et al, 2007) 5 μm thick sections were cut, deparaffinised, and subjected to antigen retrieval by microwave cooking in 0.01 M citrate buffer (pH 6.0) at 1000 W for 30 min. From here on the TMA sections and the T47D cells were treated identically. After blocking (purchased by Olink Bioscience, Uppsala, Sweden) the following primary antibodies were used: for PTK6 (BRK, anti-rabbit, sc-1188, Santa Cruz Biotech, Heidelberg, Germany, 1 : 50); for HER2 (Novocastra, anti-mouse, NCL-L-CB11, 1 : 7). The PLA protocol was followed according to the manufacturers’ instructions (Olink Bioscience, Uppsala, Sweden), with incubation of the primary antibodies at 4°C over night. Proximity ligation assay minus and PLA plus probes (containing the secondary antibodies conjugated with oligonucleotides) were added and incubated 2 h at 37°C. Afterwards, further oligonucleotides are added, allowed to hybridise to the PLA probes, and ligase joins the two hybridised oligonucleotides to a closed circle. The DNA is then amplified (rolling circle amplification), and detection of the amplicons was carried out using the ‘563 detection kit’ (including Hoechst 33342 dye nuclear staining), resulting in red fluorescence signals. The sections were mounted with Vectashield mounting media (Vector Lab., Inc., Burlingame, CA, USA).
Image acquisition and evaluation of signal counts
The hybridised slides were viewed under a confocal laser scanning microscope (AxioImager, Zeiss, Jena, Germany) equipped with the Apotome Extension (Zeiss Vision, Jena, Germany) and appropriate filters (signals: 563/581 nm, nuclei: 352/455 nm excitation/emission). Within each tissue spot optical sections were captured from three to five different tumour areas at a distance of 0.3 μm using a C-Apochromat × 63/1.2 W objective. Three-dimensional image projections were calculated (AxioVision software, Zeiss, Jena, Germany), converted to TIF format, and signals from protein complexes were evaluated using the Definiens Enterprise Image Intelligence Suite software (Definiens, Munich, Germany). A specific rule set was defined to detect and quantify semantic classes (‘signal’ and ‘nucleus’), based on fluorescence layer, intensity, and shape. Finally, for each image the mean number of protein complex signals (mean PLA) was calculated per 1000 pixels of tissue area, and subjected to statistical evaluation.
Statistics
Correlations among the mean PLA signal counts and the immunohistochemical and histopathological parameters were examined by Spearman's rank correlation test. For univariate survival analysis, Kaplan–Meier curves were calculated for distant metastasis-free survival of patients, and differences between the groups were tested with the log-rank χ2 value. Multivariate analysis was carried out using Cox proportional hazards regression and a combined stepwise selection algorithm (SAS Inst., Cary, NC, USA). All parameters reaching a significance level of P⩽0.15 in univariate analysis were offered to multivariate analysis. In all, other tests statistical significance was considered proven if P⩽0.05.
Results
Down-regulation of PTK6 and HER2 proteins in T47D cell line
The need for presence of both, HER2, and PTK6 proteins for detection of PTK6–HER2 protein complexes, was proven by using T47D cells, which slightly overexpress both, HER2 and PTK6. Proximity ligation assay was carried out after down-regulation of PTK6 and HER2, respectively, using specific small interfering RNAs (siRNAs). As shown in Figure 1, cells transfected with siRNA showed significantly reduced signal counts when compared with the non-transfected control cells. These results are corroborated by western blot analysis (Figure 1E, F) showing PTK6 and HER2 down-regulation after siRNA transfection to 21 and 49% of untreated controls, respectively.
Protein tyrosine kinase 6–HER2 complex formation in T47D cells as visualised by the PLA technique. Control T47D cells (A, C) and T47D cells after siRNA-mediated down-regulation of PTK6 (B) or HER2 (D). Western blot analysis of proteins extracted from T47D cells after transfection with siRNA targeting PTK6 (E) and HER2 (F), respectively; control cells were treated with transfection reagents only or with siRNA targeting GAPDH; (tubulin=loading control).
Visualisation and statistical evaluation of PTK6–HER2 protein complexes in tumour tissue
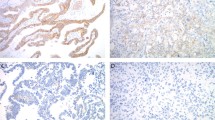
Eighty primary invasive breast carcinomas were analyzed using the PLA technique, using primary antibodies against both HER2 and PTK6 proteins. The mean number of PLA signals detected in all of the cases was compared with the data obtained by immunohistochemistry for PTK6 and HER2, and with the histopathological data and the clinical course of patients. Examples of PLA on tumour tissue specimens are shown in Figure 2 together with corresponding images from PTK6 and HER2 immunohistochemistry.
Examples for PLA on tumour tissue sections showing PLA results and the corresponding results obtained by immunohistochemical staining of PTK6 and HER2. Strong staining for HER2 (3+, A) and PTK6 (2+, B) resulted in high signal counts of protein complexes (C); weak positivity for both proteins (HER2: 1+, D; PTK6: 1+, E) resulted in low signal count (F). Further examples are given for HER2 high (G) / PTK6 low (H) expression and for HER2 low (J) / PTK6 high (K) expression, both resulting in rare PLA signals (I, L).
From the histopathological parameters (tumour size, lymphnode status, histological grade, oestrogen, and progesterone receptor status), only tumour size was significantly correlated (P=0.015) with the mean number of PLA signals. The IHC from HER2 (P=0.19) and PTK6 (P=0.1) were not significantly associated with the PLA signal counts.
With respect to the distant metastasis-free survival (univariate) the mean PLA signals showed a significant univariate association with distant metastasis-free survival of patients. As shown in Figure 3, low signal counts (⩽0.5 per 1000 pixels of tumour area, n=48 patients) were associated with better prognosis with 20.8% of patients with distant metastasis, whereas from the patients with >0.5 signals (n=28) 50% suffer from metastasis (P=0.012). Furthermore, IHC-PTK6 (P=0.018, inverse), tumour size (P=0.01), and lymph node status (P=0.004) were also significant in univariate analysis, whereas no significance level was reached by IHC-HER2 (P=0.35), ER (P=0.9), and PrR (P=0.36).
Kaplan–Meier survival curves illustrating probability of distant metastasis-free survival of breast cancer patients according to their HER2–PTK6 protein complex status. Patients with low PLA signals (⩽0.5, solid line) are associated with better prognosis (21% of patients developed distant metastases), whereas from patients with higher PLA signal counts (>0.5, dashed line) 50% suffer from distant metastases. (Mean PLA was defined as mean number of PLA signals per 1000 pixels of tumour area in the section).
Although the number of tumours is very small, we carried out multivariate analysis offering all parameters significant in univariate analysis: the prognostic value of the lymphnode status (P=0.008) was further improved by IHC-PTK6 (P=0.006) and by the mean PLA signals (P=0.01).
Discussion
The function of PTK6 remains poorly understood. Protein tyrosine kinase 6 has been linked to cell differentiation, growth regulation, and apoptosis (Harvey and Crompton, 2003; Brauer and Tyner, 2010), and there is increasing evidence that it interacts with HER receptors (Kamalati et al, 1996, 2000; Chen et al, 2004; Haegebarth et al, 2005; Aubele et al, 2008; Xiang et al, 2008) and downstream signalling molecules (Zhang et al, 2005; Ostrander et al, 2007; Aubele et al, 2008). So far, PTK6 interacting proteins were identified in in vitro studies using immunoprecipitation and western blot analysis. In breast cancer tissue significant correlation was identified between the expression levels of PTK6 and HER2 (Born et al, 2005; Aubele et al, 2007, 2008; Xiang et al, 2008). Thus, potentially, PTK6 may represent a therapeutic target in clinical management of breast cancer in addition to the established anti-HER2 therapy with the humanised antibody trastuzumab, which targets HER2, but progression is common within 1 year (Nahta and Esteva, 2007).
The PLA technique enables accurate visualisation, subcellular localisation, and quantitative evaluation of protein–protein interactions, as, for example, was shown for complexes of prostate specific antigen with α1-protease inhibitor (Zhu et al, 2009), and for EGF receptor–HER2 complex formation in vitro (Leuchowius et al, 2009).
In this study, we used the PLA technique to show for the first time ever that PTK6 forms protein complexes with HER2 in paraffin tissues from invasive breast carcinomas. Our results on tumour tissues are corroborated by down-regulation of each of these proteins in T47D cell line, resulting in reduced PLA signal counts. In tumour tissues, we identified significant correlation of PLA signals with tumour size and with distant metastasis-free survival of patients. In the context of our previous findings, in which PTK6 expression was shown to be linked to a better prognosis, the correlation of PTK6–HER2 protein complexes with occurrence of metastasis seems to be inconsistent. However, the associations of HER2 with bad prognosis (P=0.35) and of PTK6 with good prognosis (P=0.018, inverse correlation) were confirmed in this study. The association of the mean PLA signals with bad prognosis may be explained by the fact that PLA signals do not necessarily reflect the expression of the single proteins, but represent a feature independent from the single protein expressions.
Our findings show that HER2–PTK6 protein complexes are of prognostic value and that PTK6 may be considered as additional breast cancer biomarker and potential target for anticancer therapy.
Change history
29 March 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Aubele M, Auer G, Walch AK, Munro A, Atkinson MJ, Braselmann H, Fornander T, Bartlett JM (2007) PTK (protein tyrosine kinase)-6 and HER2 and 4, but not HER1 and 3 predict long-term survival in breast carcinomas. Br J Cancer 96: 801–807
Aubele M, Walch AK, Ludyga N, Braselmann H, Atkinson MJ, Luber B, Auer G, Tapio S, Cooke T, Bartlett JM (2008) Prognostic value of protein tyrosine kinase 6 (PTK6) for long-term survival of breast cancer patients. Br J Cancer 99: 1089–1095
Born M, Quintanilla-Fend L, Braselmann H, Reich U, Richter M, Hutzler P, Aubele M (2005) Simultaneous over-expression of the Her2/neu and PTK6 tyrosine kinases in archival invasive ductal breast carcinomas. J Pathol 205: 592–596
Brauer PM, Tyner AL (2010) Building a better understanding of the intracellular tyrosine kinase PTK6 – BRK by BRK. Biochem Biophys Acta 1806 (1): 66–73
Chen HY, Shen CH, Tsai YT, Lin FC, Huang YP, Chen RH (2004) Brk activates rac1 and promotes cell migration and invasion by phosphorylating paxillin. Mol Cell Biol 24: 10558–10572
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19: 403–410
Haegebarth A, Nunez R, Tyner AL (2005) The intracellular tyrosine kinase Brk sensitizes non-transformed cells to inducers of apoptosis. Cell Cycle 4: 1239–1246
Harvey AJ, Crompton MR (2003) Use of RNA interference to validate Brk as a novel therapeutic target in breast cancer: Brk promotes breast carcinoma cell proliferation. Oncogene 22: 5006–5010
Harvey AJ, Pennington CJ, Porter S, Burmi RS, Edwards DR, Court W, Eccles SA, Crompton MR (2009) Brk protects breast cancer cells from autophagic cell death induced by loss of anchorage. Am J Pathol 175 (3): 1226–1234
Kamalati T, Jolin HE, Fry MJ, Crompton MR (2000) Expression of the BRK tyrosine kinase in mammary epithelial cells enhances the coupling of EGF signalling to PI 3-kinase and Akt, via erbB3 phosphorylation. Oncogene 19: 5471–5476
Kamalati T, Jolin HE, Mitchell PJ, Barker KT, Jackson LE, Dean CJ, Page MJ, Gusterson BA, Crompton MR (1996) Brk, a breast tumor-derived non-receptor protein-tyrosine kinase, sensitizes mammary epithelial cells to epidermal growth factor. J Biol Chem 271: 30956–30963
Leuchowius K-J, Weibrecht I, Landegren U, Gedda L, Söderberg O (2009) Flow cytometric in situ proximity ligation analysis of protein interactions and post-translational modification of the epidermal growth factor receptor family. Cytometry Part A 75A: 833–839
Liu L, Gao Y, Qiu H, Miller WT, Poli V, Reich NC (2006) Identification of STAT3 as a specific substrate of breast tumor kinase. Oncogene 25: 4904–4912
Nahta R, Esteva FJ (2007) Trastuzumab: triumphs and tribulations. Oncogene 26: 3637–3643
Ostrander JH, Daniel AR, Lofgren K, Kleer CG, Lange CA (2007) Breast tumor kinase (protein tyrosine kinase 6) regulates heregulin-induced activation of ERK5 and p38 MAP kinases in breast cancer cells. Cancer Res 67: 4199–4209
Söderberg O, Gullberg M, Jarvius M, Ridderstråle K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U (2006) Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods 3 (12): 995–1000
Weaver AM, Silva CM (2007) Signal transducer and activator of transcription 5b: a new target of breast tumor kinase/protein tyrosine kinase 6. Breast Cancer Res 9: R79
Xiang B, Chatti K, Qui H, Lakshmi B, Krasnitz A, Hicks J, Yu M, Miller WT, Muthuswamy SK (2008) Brk is coamplified with ErbB2 to promote proliferation in breast cancer. PNAS 105 (34): 12463–12468
Zhang P, Ostrander JH, Faivre EJ, Olsen A, Fitzsimmons D, Lange CA (2005) Regulated association of protein kinase B/Akt with breast tumor kinase. J Biol Chem 280: 1982–1991
Zhu L, Koistinen H, Landegren U, Stenman U-H (2009) Proximity ligation measurement of the complex between prostate specific antigen and α1-prostate inhibitor. Clin Chem 55 (9): 1665–1671
Acknowledgements
Ethical approval for this study was obtained from the Ethics Committee of the Medizinische Fakultät der Technischen Universität, Munich, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Aubele, M., Spears, M., Ludyga, N. et al. In situ quantification of HER2–protein tyrosine kinase 6 (PTK6) protein–protein complexes in paraffin sections from breast cancer tissues. Br J Cancer 103, 663–667 (2010). https://doi.org/10.1038/sj.bjc.6605836
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6605836
Keywords
This article is cited by
-
Preclinical PET imaging of EGFR levels: pairing a targeting with a non-targeting Sel-tagged Affibody-based tracer to estimate the specific uptake
EJNMMI Research (2016)
-
uPAR enhances malignant potential of triple-negative breast cancer by directly interacting with uPA and IGF1R
BMC Cancer (2016)
-
In situ detection of HER2:HER2 and HER2:HER3 protein–protein interactions demonstrates prognostic significance in early breast cancer
Breast Cancer Research and Treatment (2012)
-
Analyzing human epidermal growth factor receptor family dimerization and activation using Duolink®
Nature Methods (2010)