Abstract
Background:
Although multidrug resistance protein 2 (MRP2) confers chemoresistance in some cancer types, its implication on oesophageal squamous cell carcinoma (ESCC) remains unclear.
Methods:
We evaluated MRP2 expression by immunohistochemistry and RT–PCR using 81 resected specimens from ESCC patients who did or did not receive neo-adjuvant chemotherapy (NACT), including 5-fluorouracil, doxorubicin, and cisplatin (CDDP). Correlation between MRP2 expression and response to chemotherapy was also examined in 42 pre-therapeutic biopsy samples and eight ESCC cell lines.
Results:
MRP2-positive immunostaining was more frequently observed in ESCCs with NACT than in those without NACT (27.3 vs 5.4%). The MRP2-positive patients showed poorer prognosis than MRP2-negative patients (5-year survival rate, 25.6 vs 55.7%). Concordantly, ESCC with NACT showed 2.1-fold higher mRNA expression of MRP2 than those without NACT (P=0.0350). In pre-therapeutic biopsy samples of patients with NACT, non-responders showed 2.9-fold higher mRNA expression of MRP2 than responders (P=0.0035). Among the panel of ESCC cell lines, TE14 showed the highest MRP2 mRNA expression along with the strongest resistance to CDDP. Inhibition of MRP2 expression by small-interfering RNA reduced chemoresistance to CDDP.
Conclusion:
Our data suggested that MRP2 is one of molecules, which regulate the sensitivity to chemotherapy including CDDP in advanced ESCC patients.
Similar content being viewed by others
Main
Oesophageal squamous cell carcinoma (ESCC) is the major histological form of oesophageal cancer in East Asian countries. It is one of the most lethal malignancies of the digestive tract and in most cases the initial diagnosis is established only once the malignancy is in the advanced stage (Shimada et al, 2003). Multimodal therapies are therefore necessary to prolong the survival of ESCC patients. Chemotherapy has become the standard first-line therapy for advanced ESCC patients, especially neo-adjuvant chemotherapy (NACT) (Tamoto et al, 2004). However, the initial response rate for NACT remains at 35–66% (Ajani et al, 1992; Iizuka et al, 1992; Hilgenberg et al, 1988; Ilson et al, 1998, 1999; Millar et al, 2005) and non-responders risk serious adverse effects without achieving any survival benefit.
The effectiveness of chemotherapy is often limited by drug-resistance factors in the tumours themselves. In fact, some tumours are intrinsically resistant to many kinds of chemotherapeutic agents, whereas other tumours, initially sensitive, often recur or become resistant not only to the initial agents used but also to those used subsequently. These two types of chemoresistance, intrinsic and acquired, are clinically serious problems in many types of cancer including ESCC; however, the molecular mechanisms underlying this resistance are not fully understood. More investigation into the mechanisms of chemoresistance in ESCC is needed with the goal of identifying novel predictive markers that can accurately identify non-responders before the administration of chemotherapy, thus enabling personalised therapies in ESCC patients.
Several members of the ATP-binding cassette (ABC) transporter superfamily have an important role in drug resistance in tumour cell models as well as in the clinic (Lage, 2003). These transporters mediate the ATP-dependent cellular efflux of chemotherapeutic drugs. Of the 48 human ABC transporters, multidrug resistance protein 2 (MRP2; also designated as ABCC2 or cMOAT) is expressed in the hepatocyte canalicular membrane (Kool et al, 1997), in which it functions as the major exporter of organic anions from the liver into the bile (Wada et al, 1998). Multidrug resistance protein 2 is also expressed in the kidney, gall bladder, small intestine, colon, and lung (Surowiak et al, 2006). Interestingly, several cisplatin (CDDP)-resistant human cancer cell lines overexpress MRP2, including ovarian cancer, hepatocellular carcinoma, bladder cancer, and colon cancer (Taniguchi et al, 1996; Kool et al, 1997; Liedert et al, 2003; Materna et al, 2005). In vitro data also implicated MRP2 in multidrug resistance (MDR) mechanisms during chemotherapy in some cancer cell lines (Koike et al, 1997; Materna et al, 2006; Ma et al, 2009). However, few studies have investigated MRP2 expression in ESCC (Gan et al, 2010; Tanaka et al, 2010), and thus the relationship between MRP2 expression and chemoresistance in ESCC remains unclear. The present study examined the clinical significance of MRP2 expression and its role in intrinsic and acquired resistance to chemotherapy in ESCC patients.
Patients and methods
Patients and treatments
The present study examined samples from 81 patients with histopathologically confirmed primary thoracic oesophageal cancer who underwent surgical resection at our hospital from 1988 to 2007. Table 1 details the patient characteristics. The cohort comprised 9 female and 72 male patients, aged from 42 to 80 years (median 62 years). Sub-total oesophagectomy by right thoracotomy with two or three-field lymphadenectomy was performed in all patients. Curative resection (R0) was achieved in 75 patients (92.6%), whereas the remaining 6 (7.4%) patients underwent a non-curative resection (R1, 2). None of the patients died of post-operative complications. A total of 44 patients (54.3%) with lymph node metastasis at initial diagnosis received NACT comprising two courses of 5-fluorouracil (5-FU), CDDP, and doxorubicin (DXR) (Akita et al, 2006; Yano et al, 2006; Matsuyama et al, 2007; Makino et al, 2008, 2010). Only a few patients who showed multiple metastatic lymph nodes in the surgical specimen received a regimen of docetaxel or CDDP plus 5-FU after operation (Ando et al, 2003).
After surgery, the patients were surveyed every 3 months by physical examination and measurement of serum tumour markers, every 6 months by CT scan and abdominal ultrasonography, and every year by endoscopy until tumour recurrence was evident. Patients with tumour recurrence received chemotherapy or chemoradiotherapy as long as their systemic condition permitted. The mean overall survival (OS) was 31.6 months and mean disease-free survival was 28.3 months. The mean follow-up period after surgery was 42.9 months.
Immunohistochemical analysis
MRP2 protein accumulation was examined by immunohistochemical (IHC) staining of formalin-fixed and paraffin-embedded ESCC tissue sections (Makino et al, 2009). Briefly, after de-paraffinization in xylenes and dehydration through graded ethanol solutions; endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide for 20 min. The tissue sections were then heated at 95°C for 40 min in citrate buffer (0.05 mol l−1, pH 6.0) for antigen retrieval. The sections were then incubated with mouse monoclonal antibody to MRP2 (Clone: M2III-6, ALEXIS Biochemicals, dilution 1 : 10) for 2 h at room temperature, and antibody binding was visualised using the labeled-streptavidin biotin method. Negative controls for the IHC included omission of the primary antibody. Normal human liver tissue was used as a positive control. MRP2 staining for each ESCC sample was judged ‘positive’ when more than 10% of the cancer cells in the section were immunoreactive to MRP2, and ‘negative’ when 10% or less of the cells were positive. All slides were assessed by two observers, independently and then in conference; both were blinded to the clinico-pathological parameters.
Quantitative RT–PCR analysis
Total RNA was extracted from fresh frozen resected tumours or endoscopic biopsy samples from ESCCs patients, and from cancer cell lines using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). Complementary DNA (cDNA) was generated from 1 μg RNA in a final volume of 20 μl containing oligo-(dT)-15 primer and avian myeloblastosis virus transcriptase, using the Reverse Transcription System (Promega, Madison, WI, USA). Analysis by PCR was performed using a LightCycler, real-time monitoring thermal cycler. Reaction mixture for PCR was prepared containing 2 μl of cDNA template, 3 mmol l−1 MgCl2, and 250 nmol l−1 of primer pairs, using LightCycler FastStart DNA Master SYBR Green I (Roche Diagnostics, Mannheim, Germany). The amount of each transcript was normalised against the expression of the housekeeping gene porphobilinogen deaminase (PBGD). Standard curves were constructed with 10-fold serial dilutions of cDNA obtained from non-cancerous oesophageal mucosal cell layers of tissue samples from 10 cases as a standard mixture. The sequences of PCR primers for PBGD, MRP2 were as follows: forward primer 5′-TGTCTGGTAACGGCAATGCGGCTGCAAC-3′, reverse primer 5′-TCAATGTTGCCACCACACTGTCCGTCT-3′ used for amplification of PBGD, forward primer 5′-TAATGGTCCTAGACAACGGG-3′, reverse primer 5′-GGGCCTTCTGCTAGAATTT-3′ for MRP2. The PCR cycling condition was set as follows: an initial denaturing step at 95°C for 10 min and 40 cycles at 95°C for 15 s, 58°C for 10 s, and 72°C for 25 s. The relative amount of cDNA in each sample was measured by interpolation on the standard curve, and then the relative ratio of MRP2/PBGD mRNA expression in log2 scale was calculated for each ESCC sample.
Knockdown analysis using MRP2-siRNAs
Two small-interfering RNA (siRNA-1, -2) of MRP2 (HSS102057, HSS174719) and negative control (NC) (Medium GC duplex of stealth RNAi NC duplexes) were purchased from Invitrogen. Among the eight ESCC cell lines supplied by RIKEN cell bank (Tsukuba, Japan), TE14 cells showed the highest MRP2 mRNA expression and were subsequently transfected with 15 nmol l−1 siRNA using Lipofectamine RNAiMAX (Invitrogen) in Opti-MEM I Reduced Serum Medium (Invitrogen). After 24 h, the medium was replaced by standard medium, and then 96 h from the siRNA administration, cells were collected for the following growth inhibitory assay as described below.
Growth inhibitory assay
Cells (TE14, 1 × 104 cells per well) were added in triplicate to a 96-well microplate, and after overnight incubation, the medium was replaced with 100 μl of fresh medium containing various concentrations of DXR and CDDP, both of which chemoagents have been reported to be transported by MRP2 in some types of cell lines. The TE14 cells suspended in complete medium were used as a control for cell viability. After 4h (DXR and CDDP) treatment, the cells were washed with fresh medium. The number of viable cells was assessed by the 3-(4-, 5-dimethylthiazol-2-yl)-2, 5-dyphenyl tetrazolium bromide (MTT) (Sigma, St Louis, MO, USA) assay. Briefly, 10 μl (50 μg) of MTT were added to each well after 48 h (DXR and CDDP) from the chemoadministration. The plate was incubated for 4 h at 37°C, followed by removal of medium and the addition of 100 μl of 2-propanol to each well to dissolve the resultant formazan crystals. Plate absorbance was measured in a microplate reader at a wavelength of 650 nm. After a pulsed exposure, the IC50 was calculated as percentage of control cultures that were not exposed to chemoagents using an interpolated logarithmic concentration curve. Results were derived from three independent sets of triplicate experiments.
Statistical analysis
Data are expressed as mean±s.d. Correlations between MRP2 expression and various clinico-pathological parameters were each evaluated by the χ2 test and Fisher's exact probability test. Differences in continuous parameters between two groups were evaluated by the Mann–Whitney's U-test. Prognostic variables were assessed by log-lank test, and OS was analysed by Kaplan–Meier method. These analyses were carried out using SPSS for Windows v10 (SPSS, Chicago, IL, USA). A P-value of less than 0.05 denoted the presence of statistical significance.
Results
MRP2 protein expression by immunohistochemistry in ESCC and its correlation with clinico-pathological parameters
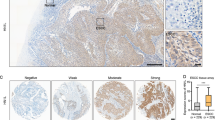
A total of 81 samples that contained both cancerous and non-cancerous lesions were evaluated for MRP2 protein expression by IHC analysis. As a positive control, liver tissue showed strong MRP2 immunostaining mainly in the hepatocyte plasma membrane (Figure 1A). No normal squamous epithelium showed significant levels of immunostaining (Figure 1B). Of all samples, 14 (17.3%) showed positive MRP2 expression, mainly in the cell membrane and cytoplasm of tumour cells (Figure 1C), whereas the remaining 67 (82.7%) were negative for MRP2 expression (Figure 1D). The positive staining was almost homogeneous in single-cancer nests and among different areas (surface, central, and deepest areas) of the cancer lesion.
MRP2 expression by immunohistochemistry. (A) Strong MRP2 expression in liver tissue as a positive control (magnification, × 400). (B) Representative normal squamous epithelium negative for MRP2 expression (magnification, × 200). (C) Representative MRP2-positive ESCC showing staining mainly in the membrane and cytoplasm of tumour cells (magnification, × 200). (D) Representative MRP2-negative oesophageal squamous cell carcinoma with no appreciable staining of tumour cells (magnification, × 200).
Table 1 lists the correlations between MRP2 expression and various clinico-pathological parameters. Of note, MRP2 expression was exceptional in the ESCC patients without NACT (2 out of 37, 5.4%), but was significantly more frequent in patients after NACT (12 out of 44, 27.3%). Women tended to have a higher rate of MRP2 expression than men (44.4 vs 13.9%, respectively), although the difference was small. Other clinico-pathological parameters including age, histological type, tumour location, pT, pN, and pStage were not associated with MRP2 expression.
Disease recurrence after curative resection was diagnosed in 35 (46.7%) of 75 patients with curative resection (R0) and the mean time to recurrence was 10.5 months. A total of 35 (43.2%) patients died and their average survival time from diagnosis to death was 1.4 years (range 0.2–4.2 years). The total 5-year OS rate was 50.9% and MRP2-positive patients showed a significantly poorer prognosis than MRP2-negative patients (5-year OS 55.7 vs 25.6%) (Figure 2).
MRP2 mRNA expressions in resected specimens and endoscopy biopsy samples
RT–PCR analysis was performed to quantify the expression of MRP2 mRNA in surgically removed specimens from 26 representative cases, including 16 with NACT and 10 without NACT. MRP2 mRNA expression in tumours with NACT was 2.1-fold higher than in those without NACT, although there was no significant difference in TNM stage and other clinico-pathological parameters between the groups (data not shown) (Figure 3A).
Differences in MRP2 mRNA expression between patients with and without neo-adjuvant chemotherapy in resected specimens (A), and between responders and non-responders at biopsy (B). (A) The relative ratio of MRP2 mRNA expression in resected tumours treated with neo-adjuvant chemotherapy (n=16) was significantly higher than in untreated cancers (n=10). (B) In endoscopy biopsy samples, the relative ratios of MRP2 mRNA expression in responders (n=22) were significantly higher than those in non-responders (n=20). Data are shown as mean±s.d. (log2 values).
The association between MRP2 mRNA expression and the effect of NACT was investigated in biopsy samples before NACT from 42 patients; the response of these patients to NACT was classified as non-responder in 22 and responder in 20. As shown in Figure 3B, MRP2 mRNA expression in non-responders was 2.9-fold higher than that in responders. Again, although these 42 samples were all advanced tumours with clinically positive lymph node metastases, there was no significant difference in clinical background parameters between the groups (data not shown).
Association between MRP2 mRNA expression and chemoresistance in ESCC cancer lines
To explore whether MRP2 expression functions specifically in chemoresistance to CDDP, we tested for a correlation between MRP2 mRNA expression and CDDP resistance (IC50) in eight ESCC cell lines (Figure 4). Relatively high MRP2 expression was observed in TE14 and TE5 cell lines, both of which displayed strong resistance to CDDP. Regression analysis showed a significant correlation between MRP2 mRNA expression and IC50 against CDDP (R=0.741, R2=0.549), suggesting that ESCC cell lines with higher MRP2 mRNA expression were more resistant to CDDP compared with those showing lower MRP2 expression.
Correlation between MRP2 mRNA expression and CDDP-resistance (IC50) in eight cell lines of ESCC. Relatively high MRP2 expression was observed in TE14 and TE5 cell lines, both of which displayed strong resistance to CDDP. Black bar: the relative ratio of MRP2 mRNA expression, grey bar: IC50 values against CDDP.
To confirm these findings by an alternative approach, we transfected MPR2 siRNAs into the TE14 line, which had the highest cellular MRP2 expression. The specific gene silencing started 48 h after the administration of siRNA (two siRNAs for MRP2 with different sequences were used: siRNA-1 and siRNA-2) and continued for 144 h, which was examined by quantitative PCR, resulting in 63.8% (siRNA-1) and 65.9% (siRNA-2) of peak MRP2 downregulation compared with NCs. The knockdown effect was stable during this period. As shown in Table 2, downregulation of MRP2 conferred increased sensitivity to CDDP, but not to DXR. IC50 values against CDDP were significantly lower in TE14 cell lines transfected with siRNA-1 and siRNA-2 compared with those transfected with NC (20.5±1.4, 17.8±1.2 vs 32.4±1.2 μ M, (siRNA-1 vs NC); P=0.0003, (siRNA-2 vs NC); P=0.0005). On the other hand, IC50 values of DXR were almost similar among TE14 cells transfected with siRNA-1, siRNA-2, and NC (5.8±0.47, 5.4±0.54 vs 6.2±0.16 μ M, (siRNA-1 vs NC); P=0.2869, (siRNA-2 vs NC); P=0.2285).
Discussion
To our knowledge, this study is the first to identify the clinical significance of MRP2 expression in chemoresistance in ESCC. Such a relationship was strongly suggested by the findings that (1) MRP2 expression in the clinical biopsy samples before NACT was significantly negatively correlated with the effect of NACT, and (2) in the cultured cell line, artificial MRP2 downregulation resulted in increased resistance to the chemotherapy. Furthermore, the clinical samples of patients treated with NACT showed significantly higher expression of MRP2 at both the protein and mRNA levels than those without NACT, and the increased MRP2 expression was associated with poor prognosis. Although complicated, these clinical observations implicated MRP2 in the acquired resistance to chemotherapy commonly encountered in ESCC patients.
Intrinsic or acquired drug resistance is a major factor limiting the effectiveness of chemotherapy in various cancers including ESCC. Drug resistance by tumours occurs not only to a single cytotoxic agent but also in the form of cross-resistance to many agents called MDR. One of the major mechanisms of MDR is an increased ability of tumour cells to actively efflux drugs, decreasing the intracellular drug accumulation. This mechanism is mediated by ATP-dependent drug efflux pumps known as ABC transporters (Leonard et al, 2003; Ozben, 2006). To date, at least 48 human ABC transporters have been identified, and they have been divided into seven sub-families, ABC-A through ABC-G. The first ABC transporter identified in this context was P-glycoprotein (PgP, MDR1, ABCB1) (Kartner et al, 1983), and in the absence of overexpressed MDR1, the protein MRP1, ABCC1 was discovered because of the MDR phenotype (Cole et al, 1992).
Cisplatin resistance is not a feature of MDR phenotypes conferred by either MDR1 or MRP1 (Borst et al, 2000). The finding that ABC transporter MRP2 could mediate active efflux of CDDP conjugated to glutathione (Taniguchi et al, 1996), supported by evidence that intracellular glutathione levels were related to CDDP toxicity (Ozols, 1985), suggested a possible role for active efflux as a resistance mechanism. In addition, human carcinoma cell line studies showed increased levels of MRP2 mRNA associated with relative CDDP resistance, decreased intracellular accumulation of CDDP, and decreased DNA adduct formation (Kool et al, 1997; Liedert et al, 2003). In ESCC cell lines (TE2, TE13) Tanaka et al (2010), in their analysis of the intracellular localisation of CDDP by using in-air micro-particle induced X-ray emission, recently reported that TE2 cells, which express lower MRP2 than TE13, had higher intracellular CDDP concentrations and sensitivity than TE13 cells. This is also in agreement with our present in vitro data regarding CDDP. In human tissue samples, accumulating evidence indicates that MRP2 expression is also associated with intrinsic CDDP resistance in the clinical setting, using tissues obtained from patients with colorectal cancer (Hinoshita et al, 2000), small-cell lung carcinoma (Ushijima et al, 2007), and ovarian cancer (Surowiak et al, 2006). These results are also consistent with our data from cancer tissue samples, although our in vitro data involving each single agent could not necessarily be translated directly to a clinical response to combination chemotherapy because of possible synergistic effects. However, in contrast with these data, other studies failed to show a significant association between MRP2 expression and chemosensitivity in patients with ovarian cancer (Arts et al, 1999; Materna et al, 2004) or lung cancer (Filipits et al, 2007; Kim et al, 2009). It therefore seems likely that multiple factors such as drug accumulation, DNA repair capacity, and apoptotic sensitivity contribute to clinical tumour chemosensitivity, and a mechanistic relationship could be difficult to detect amongst an unselected patient cohort in which a number of other factors also affect clinical outcome. As clinical significance of MRP2 other than chemosensitivity, Gan et al (2010) reported that MRP2 expression was significantly higher in poorly differentiated ESCC tumours compared with moderate or well differentiated ones, which was not observed in our study.
In terms of the contribution to acquired chemoresistance, the present IHC and qRT–PCR data showed higher MRP2 expression in resected tumours with NACT compared with those without NACT, implying residual tumours after NACT acquired the feature of chemoresistance. Unfortunately, we could not compare MRP2 expression levels in cancer tissues from the same patient before and after NACT because no samples were available. Nooter et al (1998) reported significantly higher MRP expression, although not specific MRP2 expression, in ESCC tumours from non-responders to CDDP-based chemotherapy when comparing MRP levels in paired tumour samples before and after chemotherapy, suggesting that chemotherapy was selected for drug-resistant cell clones. Furthermore, other in vitro analyses by Noma et al (2008) established two CDDP-resistant pancreatic cancer cell lines (SUIT-2-CD3 and SUIT-2-CD4) by continuously administering 10 nM CDDP for 3 and 4 months, respectively. Results of RT–PCR indicated that induction of MRP2 mRNA expression was significantly increased by 1.5- and 2.5-fold in SUIT-2-CD3 and SUIT-2-CD4 cells, respectively, compared with parent cells, whereas MRP1 and MRP3 expression remained unchanged, implying a contribution of MRP2 to acquired resistance for CDDP in pancreatic cancer.
An important observation regarding the functional significance of MRP2 expressed in tumour cells could be the sub-cellular localisation. In normal tissues, MRP2 is expressed in functionally polarised cells in which it specifically localises to the apical membrane of these cells. Apical localisation has also been described in tumours arising from these sites, a feature attributed to a targeting signal in the C-terminus of the MRP2 molecule (Harris et al, 2001). Single-nucleotide polymorphisms in MRP2 have been described that result in cytoplasmic localisation of the protein and that may reduce in vivo function (Hirouchi et al, 2004). Reduced CDDP sensitivity has also been reported in polarised mammalian kidney cells transfected with appropriately localised MRP2 (Cui et al, 1999). Furthermore, data of Surowiak et al (2006) indicated that MRP2 could confer resistance to CDDP in ovarian carcinoma only when expressed at the nuclear membrane, and this was supported by in vitro data (Materna et al, 2006). Although our IHC results showed MRP2-positive staining of both cytoplasm and membrane in tumour cells, MRP2 protein located in the cell cytoplasm might not function as an efflux pump (Evers et al, 1998). Further analysis focusing on the sub-cellular localisation of MRP2, and on the functional and clinical significance of such cellular location, is needed to elucidate the specific mechanism of chemoresistance induced by MRP2 in ESCC.
In conclusion, MRP2 expression seems to be associated with intrinsic resistance to chemotherapy in patients with ESCC, and is likely to also have a role in acquired chemoresistance. Further studies with larger cohorts are warranted to verify these results prospectively. The findings of this study open the door for exploration of efficacious treatment strategies and development of new therapeutic approaches for ESCC.
Change history
29 March 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ajani JA, Ryan B, Rich TA, McMurtrey M, Roth JA, DeCaro L, Levin B, Mountain C (1992) Prolonged chemotherapy for localised squamous carcinoma of the oesophagus. Eur J Cancer 28A: 880–884
Akita H, Doki Y, Miyata H, Hirao T, Yano M, Takachi K, Miyashiro I, Sasaki Y, Ishikawa O, Ohigashi H, Imaoka S (2006) Clinical significance of the second cycle response to cisplatin-based chemotherapy as preoperative treatment for esophageal squamous cell carcinoma. J Surg Oncol 93: 401–409
Ando N, Iizuka T, Ide H, Ishida K, Shinoda M, Nishimaki T, Takiyama W, Watanabe H, Isono K, Aoyama N, Makuuchi H, Tanaka O, Yamana H, Ikeuchi S, Kabuto T, Nagai K, Shimada Y, Kinjo Y, Fukuda H (2003) Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study – JCOG9204. J Clin Oncol 21: 4592–4596
Arts HJ, Katsaros D, de Vries EG, Massobrio M, Genta F, Danese S, Arisio R, Scheper RJ, Kool M, Scheffer GL, Willemse PH, van der Zee AG, Suurmeijer AJ (1999) Drug resistance-associated markers P-glycoprotein, multidrug resistance-associated protein 1, multidrug resistance-associated protein 2, and lung resistance protein as prognostic factors in ovarian carcinoma. Clin Cancer Res 5: 2798–2805
Borst P, Evers R, Kool M, Wijnholds J (2000) A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst 92: 1295–1302
Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG (1992) Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258: 1650–1654
Cui Y, Konig J, Buchholz JK, Spring H, Leier I, Keppler D (1999) Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol Pharmacol 55: 929–937
Evers R, Kool M, van Deemter L, Janssen H, Calafat J, Oomen LC, Paulusma CC, Oude Elferink RP, Baas F, Schinkel AH, Borst P (1998) Drug export activity of the human canalicular multispecific organic anion transporter in polarized kidney MDCK cells expressing cMOAT (MRP2) cDNA. J Clin Invest 101: 1310–1319
Filipits M, Haddad V, Schmid K, Huynh A, Dunant A, Andre F, Brambilla E, Stahel R, Pignon JP, Soria JC, Popper HH, Le Chevalier T, Pirker R (2007) Multidrug resistance proteins do not predict benefit of adjuvant chemotherapy in patients with completely resected non-small cell lung cancer: International Adjuvant Lung Cancer Trial Biologic Program. Clin Cancer Res 13: 3892–3898
Gan SY, Zhong XY, Xie SM, Li SM, Peng H, Luo F (2010) Expression and significance of tumor drug resistance related proteins and beta-catenin in esophageal squamous cell carcinoma. Chin J Cancer 29: 300–305
Harris MJ, Kuwano M, Webb M, Board PG (2001) Identification of the apical membrane-targeting signal of the multidrug resistance-associated protein 2 (MRP2/MOAT). J Biol Chem 276: 20876–20881
Hilgenberg AD, Carey RW, Wilkins Jr EW, Choi NC, Mathisen DJ, Grillo HC (1988) Preoperative chemotherapy, surgical resection, and selective postoperative therapy for squamous cell carcinoma of the esophagus. Ann Thorac Surg 45: 357–363
Hinoshita E, Uchiumi T, Taguchi K, Kinukawa N, Tsuneyoshi M, Maehara Y, Sugimachi K, Kuwano M (2000) Increased expression of an ATP-binding cassette superfamily transporter, multidrug resistance protein 2, in human colorectal carcinomas. Clin Cancer Res 6: 2401–2407
Hirouchi M, Suzuki H, Itoda M, Ozawa S, Sawada J, Ieiri I, Ohtsubo K, Sugiyama Y (2004) Characterization of the cellular localization, expression level, and function of SNP variants of MRP2/ABCC2. Pharm Res 21: 742–748
Iizuka T, Kakegawa T, Ide H, Ando N, Watanabe H, Tanaka O, Takagi I, Isono K, Ishida K, Arimori M, Endo M, Fukushima M (1992) Phase II evaluation of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japanese Esophageal Oncology Group Trial. Jpn J Clin Oncol 22: 172–176
Ilson DH, Ajani J, Bhalla K, Forastiere A, Huang Y, Patel P, Martin L, Donegan J, Pazdur R, Reed C, Kelsen DP (1998) Phase II trial of paclitaxel, fluorouracil, and cisplatin in patients with advanced carcinoma of the esophagus. J Clin Oncol 16: 1826–1834
Ilson DH, Saltz L, Enzinger P, Huang Y, Kornblith A, Gollub M, O’Reilly E, Schwartz G, DeGroff J, Gonzalez G, Kelsen DP (1999) Phase II trial of weekly irinotecan plus cisplatin in advanced esophageal cancer. J Clin Oncol 17: 3270–3275
Kartner N, Shales M, Riordan JR, Ling V (1983) Daunorubicin-resistant Chinese hamster ovary cells expressing multidrug resistance and a cell-surface P-glycoprotein. Cancer Res 43: 4413–4419
Kim YH, Ishii G, Goto K, Ota S, Kubota K, Murata Y, Mishima M, Saijo N, Nishiwaki Y, Ochiai A (2009) Expression of breast cancer resistance protein is associated with a poor clinical outcome in patients with small-cell lung cancer. Lung Cancer 65: 105–111
Koike K, Kawabe T, Tanaka T, Toh S, Uchiumi T, Wada M, Akiyama S, Ono M, Kuwano M (1997) A canalicular multispecific organic anion transporter (cMOAT) antisense cDNA enhances drug sensitivity in human hepatic cancer cells. Cancer Res 57: 5475–5479
Kool M, de Haas M, Scheffer GL, Scheper RJ, van Eijk MJ, Juijn JA, Baas F, Borst P (1997) Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res 57: 3537–3547
Lage H (2003) ABC-transporters: implications on drug resistance from microorganisms to human cancers. Int J Antimicrob Agents 22: 188–199
Leonard GD, Fojo T, Bates SE (2003) The role of ABC transporters in clinical practice. Oncologist 8: 411–424
Liedert B, Materna V, Schadendorf D, Thomale J, Lage H (2003) Overexpression of cMOAT (MRP2/ABCC2) is associated with decreased formation of platinum-DNA adducts and decreased G2-arrest in melanoma cells resistant to cisplatin. J Invest Dermatol 121: 172–176
Ma JJ, Chen BL, Xin XY (2009) Inhibition of multi-drug resistance of ovarian carcinoma by small interfering RNA targeting to MRP2 gene. Arch Gynecol Obstet 279: 149–157
Makino T, Doki Y, Miyata H, Yasuda T, Yamasaki M, Fujiwara Y, Takiguchi S, Higuchi I, Hatazawa J, Monden M (2008) Use of (18)F-fluorodeoxyglucose-positron emission tomography to evaluate responses to neo-adjuvant chemotherapy for primary tumor and lymph node metastasis in esophageal squamous cell carcinoma. Surgery 144: 793–802
Makino T, Miyata H, Yamasaki M, Fujiwara Y, Takiguchi S, Nakajima K, Higuchi I, Hatazawa J, Mori M, Doki Y (2010) Utility of response evaluation to neo-adjuvant chemotherapy by (18)F-fluorodeoxyglucose-positron emission tomography in locally advanced esophageal squamous cell carcinoma. Surgery 148: 908–918
Makino T, Yamasaki M, Takeno A, Shirakawa M, Miyata H, Takiguchi S, Nakajima K, Fujiwara Y, Nishida T, Matsuura N, Mori M, Doki Y (2009) Cytokeratins 18 and 8 are poor prognostic markers in patients with squamous cell carcinoma of the oesophagus. Br J Cancer 101: 1298–1306
Materna V, Liedert B, Thomale J, Lage H (2005) Protection of platinum-DNA adduct formation and reversal of cisplatin resistance by anti-MRP2 hammerhead ribozymes in human cancer cells. Int J Cancer 115: 393–402
Materna V, Pleger J, Hoffmann U, Lage H (2004) RNA expression of MDR1/P-glycoprotein, DNA-topoisomerase I, and MRP2 in ovarian carcinoma patients: correlation with chemotherapeutic response. Gynecol Oncol 94: 152–160
Materna V, Stege A, Surowiak P, Priebsch A, Lage H (2006) RNA interference-triggered reversal of ABCC2-dependent cisplatin resistance in human cancer cells. Biochem Biophys Res Commun 348: 153–157
Matsuyama J, Doki Y, Yasuda T, Miyata H, Fujiwara Y, Takiguchi S, Yamasaki M, Makari Y, Matsuura N, Mano M, Monden M (2007) The effect of neoadjuvant chemotherapy on lymph node micrometastases in squamous cell carcinomas of the thoracic esophagus. Surgery 141: 570–580
Millar J, Scullin P, Morrison A, McClory B, Wall L, Cameron D, Philips H, Price A, Dunlop D, Eatock M (2005) Phase II study of gemcitabine and cisplatin in locally advanced/metastatic oesophageal cancer. Br J Cancer 93: 1112–1116
Noma B, Sasaki T, Fujimoto Y, Serikawa M, Kobayashi K, Inoue M, Itsuki H, Kamigaki M, Minami T, Chayama K (2008) Expression of multidrug resistance-associated protein 2 is involved in chemotherapy resistance in human pancreatic cancer. Int J Oncol 33: 1187–1194
Nooter K, Kok T, Bosman FT, van Wingerden KE, Stoter G (1998) Expression of the multidrug resistance protein (MRP) in squamous cell carcinoma of the oesophagus and response to pre-operative chemotherapy. Eur J Cancer 34: 81–86
Ozben T (2006) Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett 580: 2903–2909
Ozols RF (1985) Pharmacologic reversal of drug resistance in ovarian cancer. Semin Oncol 12: 7–11
Shimada H, Nabeya Y, Okazumi S, Matsubara H, Shiratori T, Gunji Y, Kobayashi S, Hayashi H, Ochiai T (2003) Prediction of survival with squamous cell carcinoma antigen in patients with resectable esophageal squamous cell carcinoma. Surgery 133: 486–494
Surowiak P, Materna V, Kaplenko I, Spaczynski M, Dolinska-Krajewska B, Gebarowska E, Dietel M, Zabel M, Lage H (2006) ABCC2 (MRP2, cMOAT) can be localized in the nuclear membrane of ovarian carcinomas and correlates with resistance to cisplatin and clinical outcome. Clin Cancer Res 12: 7149–7158
Tamoto E, Tada M, Murakawa K, Takada M, Shindo G, Teramoto K, Matsunaga A, Komuro K, Kanai M, Kawakami A, Fujiwara Y, Kobayashi N, Shirata K, Nishimura N, Okushiba S, Kondo S, Hamada J, Yoshiki T, Moriuchi T, Katoh H (2004) Gene-expression profile changes correlated with tumor progression and lymph node metastasis in esophageal cancer. Clin Cancer Res 10: 3629–3638
Tanaka N, Kimura H, Faried A, Sakai M, Sano A, Inose T, Sohda M, Okada K, Nakajima M, Miyazaki T, Fukuchi M, Kato H, Asao T, Kuwano H, Satoh T, Oikawa M, Kamiya T, Arakawa K (2010) Quantitative analysis of cisplatin sensitivity of human esophageal squamous cancer cell lines using in-air micro-PIXE. Cancer Sci 101: 1487–1492
Taniguchi K, Wada M, Kohno K, Nakamura T, Kawabe T, Kawakami M, Kagotani K, Okumura K, Akiyama S, Kuwano M (1996) A human canalicular multispecific organic anion transporter (cMOAT) gene is overexpressed in cisplatin-resistant human cancer cell lines with decreased drug accumulation. Cancer Res 56: 4124–4129
Ushijima R, Takayama K, Izumi M, Harada T, Horiuchi Y, Uchino J, Hara N, Nakanishi Y (2007) Immunohistochemical expression of MRP2 and clinical resistance to platinum-based chemotherapy in small cell lung cancer. Anticancer Res 27: 4351–4358
Wada M, Toh S, Taniguchi K, Nakamura T, Uchiumi T, Kohno K, Yoshida I, Kimura A, Sakisaka S, Adachi Y, Kuwano M (1998) Mutations in the canilicular multispecific organic anion transporter (cMOAT) gene, a novel ABC transporter, in patients with hyperbilirubinemia II/Dubin-Johnson syndrome. Hum Mol Genet 7: 203–207
Yano M, Takachi K, Doki Y, Miyashiro I, Kishi K, Noura S, Eguchi H, Yamada T, Ohue M, Ohigashi H, Sasaki Y, Ishikawa O, Imaoka S (2006) Preoperative chemotherapy for clinically node-positive patients with squamous cell carcinoma of the esophagus. Dis Esophagus 19: 158–163
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Yamasaki, M., Makino, T., Masuzawa, T. et al. Role of multidrug resistance protein 2 (MRP2) in chemoresistance and clinical outcome in oesophageal squamous cell carcinoma. Br J Cancer 104, 707–713 (2011). https://doi.org/10.1038/sj.bjc.6606071
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6606071
Keywords
This article is cited by
-
Risk stratification of oesophageal squamous cell carcinoma using change in total lesion glycolysis and number of PET-positive lymph nodes
British Journal of Cancer (2023)
-
Lung resistance-related protein (LRP) predicts favorable therapeutic outcome in Acute Myeloid Leukemia
Scientific Reports (2019)
-
Identification of MRP2 as a targetable factor limiting oxaliplatin accumulation and response in gastrointestinal cancer
Scientific Reports (2019)
-
Delineation of proapoptotic signaling of anthracene-shelled M2L4 metallacapsules and their synergistic activity with curcumin in cisplatin-sensitive and resistant tumor cell lines
Investigational New Drugs (2019)
-
Different Efflux Transporter Affinity and Metabolism of 99mTc-2-Methoxyisobutylisonitrile and 99mTc-Tetrofosmin for Multidrug Resistance Monitoring in Cancer
Pharmaceutical Research (2019)