Summary:
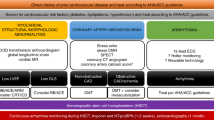
The value of pre-transplant factors for predicting the development of cardiac complications after transplantation has been inconsistent among studies. We analyzed the impact of pre-transplant factors on the incidence of severe cardiac complications in 164 hematopoietic stem cell transplant recipients. We identified eight patients (4.8%) who experienced grade III or IV cardiac complications according to the Bearman criteria. Seven died of cardiac causes a median of 3 days after the onset of cardiac complications. On univariate analysis, both the cumulative dose of anthracyclines and the use of anthracyclines within 60 days before transplantation affected the incidence of severe cardiac complications (P=0.0091 and 0.011). The dissociation of heart rate and body temperature, which reflects ‘relative tachycardia’, was also associated with a higher incidence of cardiac complications (P=0.024). None of the variables obtained by electrocardiography or echocardiography were useful for predicting cardiac complications after transplantation, although the statistical power might not be sufficient to detect the usefulness of ejection fraction. On a multivariate analysis, the cumulative dose of anthracyclines was the only independent significant risk factor for severe cardiac complications. We conclude that the cumulative dose of anthracyclines is the most potent predictor of cardiac complications and the administration of anthracyclines should be avoided within two months before transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Akahori M, Nakamae H, Hino M et al. Electrocardiogram is very useful for predicting acute heart failure following myeloablative chemotherapy with hematopoietic stem cell transplantation rescue. Bone Marrow Transplant 2003; 31: 585–590.
Bearman SI, Petersen FB, Schor RA et al. Radionuclide ejection fractions in the evaluation of patients being considered for bone marrow transplantation: risk for cardiac toxicity. Bone Marrow Transplant 1990; 5: 173–177.
Braverman AC, Antin JH, Plappert MT et al. Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol 1991; 9: 1215–1223.
Brockstein BE, Smiley C, Al-Sadir J et al. Cardiac and pulmonary toxicity in patients undergoing high-dose chemotherapy for lymphoma and breast cancer: prognostic factors. Bone Marrow Transplant 2000; 25: 885–894.
Fujimaki K, Maruta A, Yoshida M et al. Severe cardiac toxicity in hematological stem cell transplantation: predictive value of reduced left ventricular ejection fraction. Bone Marrow Transplant 2001; 27: 307–310.
Goldberg MA, Antin JH, Guinan EC et al. Cyclophosphamide cardiotoxicity: an analysis of dosing as a risk factor. Blood 1986; 68: 1114–1118.
Murdych T, Weisdorf DJ . Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977–1997. Bone Marrow Transplant 2001; 28: 283–287.
Nakamae H, Tsumura K, Hino M et al. QT dispersion as a predictor of acute heart failure after high-dose cyclophosphamide. Lancet 2000; 355: 805–806.
Hertenstein B, Stefanic M, Schmeiser T et al. Cardiac toxicity of bone marrow transplantation: predictive value of cardiologic evaluation before transplant. J Clin Oncol 1994; 12: 998–1004.
Herait P, Poutignat N, Marty M et al. Early assessment of a new anticancer drug analogue—are the historical comparisons obsolete? The French experience with pirarubicin. Eur J Cancer 1992; 28A: 1670–1676.
Casazza AM, Savi G, Pratesi G et al. Antitumor activity in mice of 4′-deoxydoxorubicin in comparison with doxorubicin. Eur J Cancer Clin Oncol 1983; 19: 411–418.
Hori S, Shirai M, Hirano S et al. Antitumor activity of new anthracycline antibiotics, aclacinomycin-A and its analogs, and their toxicity. Gann 1977; 68: 685–690.
Anderlini P, Benjamin RS, Wong FC et al. Idarubicin cardiotoxicity: a retrospective study in acute myeloid leukemia and myelodysplasia. J Clin Oncol 1995; 13: 2827–2834.
Zangari M, Henzlova MJ, Ahmad S et al. Predictive value of left ventricular ejection fraction in stem cell transplantation. Bone Marrow Transplant 1999; 23: 917–920.
Kakavas PW, Ghalie R, Parrillo JE et al. Angiotensin converting enzyme inhibitors in bone marrow transplant recipients with depressed left ventricular function. Bone Marrow Transplant 1995; 15: 859–861.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakata-Yanagimoto, M., Kanda, Y., Nakagawa, M. et al. Predictors for severe cardiac complications after hematopoietic stem cell transplantation. Bone Marrow Transplant 33, 1043–1047 (2004). https://doi.org/10.1038/sj.bmt.1704487
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704487
Keywords
This article is cited by
-
Association of persistent tachycardia with early myocardial dysfunction in children undergoing allogeneic hematopoietic cell transplantation
Bone Marrow Transplantation (2021)
-
Cardiorespiratory fitness and physical performance after childhood hematopoietic stem cell transplantation: a systematic review and meta-analysis
Bone Marrow Transplantation (2021)
-
Cardiovascular risk assessment and management of patients undergoing hematopoietic cell transplantation
Bone Marrow Transplantation (2021)
-
Cardiovascular disease and its management in children and adults undergoing hematopoietic stem cell transplantation
Journal of Thrombosis and Thrombolysis (2021)
-
Incidence and predictors of severe cardiotoxicity in patients with severe aplastic anaemia after haploidentical haematopoietic stem cell transplantation
Bone Marrow Transplantation (2019)