Summary:
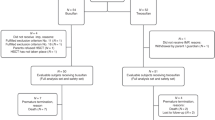
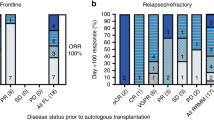
This phase I/II study evaluated high-dose treosulfan in patients with high-grade lymphoma. In all, 21 patients (median age 51, 25–60 years) with primary refractory disease (n=3) or early (n=11) or late (n=7) relapse received DexaBEAM and one course etoposide for cytoreduction and PBPC mobilization. Subsequently, 16 patients received 30 g/m2 treosulfan and 140 mg/m2 melphalan, followed by autologous transplantation. Nine patients received a 2nd high-dose treatment (HDT) with 30 g/m2 treosulfan and 750 mg/m2 thiotepa. Recovery time to >1/nl leukocytes and >25/nl thrombocytes was 8.9 (range 8–11) and 11.9 (8–16) days after 1st and 9.6 (7–13) and 13 (9–19) days after 2nd HDT. Reversible grade 3 or 4 nonhematologic toxicities included mucositis (n=7), infection (n=7) and one episode of re-entry tachycardia. Two treatment-related deaths occurred after 2nd HDT. Since three dose-limiting toxicities occurred among nine patients receiving tandem HDT, 30 g/m2 of treosulfan was considered MTD in this setting. Patients with late compared to early relapse or refractory disease had a higher probability of CR (6/7 vs 3/14 patients, P=0.017) and overall survival (71 vs 21%, P<0.05, 24–49 months follow-up). In conclusion, high-dose treosulfan as major therapy component induces sustained complete remissions in relapsed high-grade lymphoma with acceptable toxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Philip T, Guglielmi C, Hagenbeck A et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med 1995; 333: 1540–1545.
Bosly A, Gisselbrecht C . Therapy intensification with autologous transplantation in non-Hodgkin's lymphomas. Bull Cancer 2001; 88: 877–887.
Gupta V, Lazarus HM, Keating A . Myeloablative conditioning regimens for AML allografts: 30 years later. Bone Marrow Transplant 2003; 32: 969–978.
Jones RJ, Lee KS, Beschorner WE et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987; 44: 778–783.
Hänel M, Kröger N, Sonnenberg S et al. Busulfan, cyclophosphamide, and etoposide as high-dose conditioning regimen in patients with malignant lymphoma. Ann Hematol 2002; 81: 96–102.
Slattery JT, Risler LJ . Therapeutic monitoring of busulfan in hematopoietic stem cell transplantation. Ther Drug Monit 1998; 20: 543–549.
Hassan M . The role of busulfan in bone marrow transplantation. Med Oncol 1999; 16: 166–176.
De La Camera R, Thomas JF, Figuera A et al. High dose busulfan and seizures. Bone Marrow Transplant 1991; 7: 363–364.
Feit PW . Stereoisomer 1,4-di-O-methansulfonyl-butan-1,2,3,4-tetrole. Tetrahedron Lett 1961; 20: 716–717.
Fenelly J . Treosulfan (hydroxybusulfan) in ovarian carcinoma. In: Nelson JD, Grassi C (eds). Current Chemotherapy and Infectious Disease. American Society for Microbiology: Washington, DC, 1980; pp 1683–1684.
Meden H, Wittkop Y, Kuhn W . Maintenance chemotherapy with oral treosulfan following first-line treatment in patients with advanced ovarian cancer: feasibility and toxicity. Anticancer Res 1997; 17: 2221–2224.
Scheulen ME, Hilger RA, Oberhoff C et al. Clinical phase I dose escalation and pharmacokinetic study of high-dose chemotherapy with treosulfan and autologous peripheral blood stem cell transplantation in patients with advanced malignancies. Clin Cancer Res 2000; 6: 4209–4216.
Hilger RA, Harstrick A, Eberhardt W et al. Clinical pharmacokinetics of intravenous treosulfan in patients with advanced solid tumors. Cancer Chemother Pharmacol 1998; 42: 99–104.
Meinhardt G, Dayyani F, Jahrsdorfer B et al. Treosulfan is an effective inducer of cell death in myeloma cell lines and primary myeloma cells from patients. Br J Haematol 2003; 122: 892–899.
Fichtner I, Becker M, Baumgart J . Antileukaemic activity of treosulfan in xenografted human acute lymphoblastic leukaemias (ALL). Eur J Cancer 2003; 39: 801–807.
Casper J, Knauf W, Kiefer T et al. Treosulfan and fludarabine: a new toxicity-reduced conditioning regimen for allogeneic hematopoietic stem cell transplantation. Blood 2003; 103: 725–731.
Jantunen E, Kuittinen T, Nousiainen T . BEAC or BEAM for high-dose therapy in patients with non-Hodgkin's lymphoma? A single centre analysis on toxicity and efficacy. Leukemia Lymphoma 2003; 44: 1151–1158.
Shimoni A, Smith TL, Aleman A et al. Thiotepa, busulfan, cyclophosphamide (TBC) and autologous hematopoietic transplantation: an intensive regimen for the treatment of multiple myeloma. Bone Marrow Transplant 2001; 27: 821–828.
Cheng T, Forsyth P, Chaudhry A et al. High-dose thiotepa, busulfan, cyclophosphamide and ASCT without whole-brain radiotherapy for poor prognosis primary CNS lymphoma. Bone Marrow Transplant 2003; 31: 679–685.
Lee JL, Gooley T, Bensinger W et al. Veno-occlusive disease of the liver after busulfan, melphalan, and thiotepa conditioning therapy: incidence, risk factors, and outcome. Biol Blood Marrow Transplant 1999; 5: 306–315.
Reiser M, Jostin A, Draube A et al. Successful peripheral blood stem cell mobilization with etoposide in patients with relapsed or resistant lymphoma who failed cyclophosphamide mobilization. Bone Marrow Transplant 1999; 23: 1223–1228.
Junghanss C, Leithäuser M, Wilhem S et al. High-dose etoposide phosphate and G-CSF mobilizes peripheral blood stem cells in patients that previously failed to mobilize. Ann Hematol 2001; 80: 96–102.
Moskowitz CH, Bertino JR, Glassman JR et al. Ifosfamide, carboplatin, and etoposide: a highly effective cytoreduction and peripheral-blood progenitor-cell mobilization regimen for transplant-eligible patients with non-Hodgkin's lymphoma. J Clin Oncol 1999; 17: 3776–3785.
Bolwell BJ, Kalaycio M, Sobecks R et al. A multivariable analysis of factors influencing mucositis after autologous progenitor cell transplantation. Bone Marrow Transplant 2002; 30: 587–591.
Guglielmi C, Gomez F, Philip T et al. Time to relapse has prognostic value in patients with aggressive lymphoma enrolled in the PARMA trial. J Clin Oncol 1998; 16: 3264–3269.
Vose JM, Zhang MJ, Rowlings PA et al. Autologous transplantation for diffuse aggressive non-Hodgkin's lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol 2001; 19: 406–413.
Seropian S, Bahceci E, Cooper DL . Allogeneic peripheral blood stem cell transplantation for high-risk non-Hodgkin's lymphoma. Bone Marrow Transplant 2003; 32: 763–769.
Bierman PJ, Sweetenham JW, Loberiza FR et al. Syngeneic hematopoietic stem-cell transplantation for non-Hodgkin's lymphoma: a comparison with allogeneic and autologous transplantation – The Lymphoma Working Committee of the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation. J Clin Oncol 2003; 21: 3744–3753.
Mollee P, Lazarus HM, Lipton J . Why aren’t we performing more allografts for aggressive non-Hodgkin's lymphoma? Bone Marrow Transplant 2003; 31: 953–960.
Gisselbrecht C, Mounier N . Rituximab: enhancing outcome of autologous stem cell transplantation in non-Hodgkin's lymphoma. Semin Oncol 2003; 30: 28–33.
Benekli M, Hahn T, Shafi F et al. Effect of rituximab on peripheral blood stem cell mobilization and engraftment kinetics in non-Hodgkin's lymphoma patients. Bone Marrow Transplant 2003; 32: 139–143.
Shimoni A, Hardan I, Avigdor A et al. Rituximab reduces relapse risk after allogeneic and autologous stem cell transplantation in patients with high-risk aggressive non-Hodgkin's lymphoma. Br J Haematol 2003; 122: 457–464.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koenigsmann, M., Mohren, M., Jentsch-Ullrich, K. et al. High-dose treosulfan in patients with relapsed or refractory high-grade lymphoma receiving tandem autologous blood stem cell transplantation. Bone Marrow Transplant 34, 477–483 (2004). https://doi.org/10.1038/sj.bmt.1704626
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704626
Keywords
This article is cited by
-
Treosulfan plus fludarabine versus TEAM as conditioning treatment before autologous stem cell transplantation for B-cell Non-Hodgkin lymphoma
Bone Marrow Transplantation (2022)
-
Risk-adapted, treosulfan-based therapy with auto- and allo-SCT for relapsed/refractory aggressive NHL: a prospective phase-II trial
Bone Marrow Transplantation (2014)
-
Long-term follow-up of children conditioned with Treosulfan: German and Austrian experience
Bone Marrow Transplantation (2013)
-
Preclinical studies of treosulfan demonstrate potent activity in Ewing’s sarcoma
Cancer Chemotherapy and Pharmacology (2008)