Abstract
Avicins, a family of plant triterpene electrophiles, can trigger apoptosis-associated tumor cell death, and suppress chemical-induced carcinogenesis by its anti-inflammatory, anti-mutagenic, and antioxidant properties. Here, we show that tumor cells treated with benzyloxycarbonylvalyl-alanyl–aspartic acid (O-methyl)–fluoro-methylketone, an apoptosis inhibitor, and Bax−/−Bak−/− apoptosis-resistant cells can still undergo cell death in response to avicin D treatment. We demonstrate that this non-apoptotic cell death is mediated by autophagy, which can be suppressed by chloroquine, an autophagy inhibitor, and by specific knockdown of autophagy-related gene-5 (Atg5) and Atg7. Avicin D decreases cellular ATP levels, stimulates the activation of AMP-activated protein kinase (AMPK), and inhibits mammalian target of rapamycin (mTOR) and S6 kinase activity. Suppression of AMPK by compound C and dominant-negative AMPK decreases avicin D-induced autophagic cell death. Furthermore, avicin D-induced autophagic cell death can be abrogated by knockdown of tuberous sclerosis complex 2 (TSC2), a key mediator linking AMPK to mTOR inhibition, suggesting that AMPK activation is a crucial event targeted by avicin D. These findings indicate the therapeutic potential of avicins by triggering autophagic cell death.
Similar content being viewed by others
Main
Autophagy, an evolutionarily conserved process by which stressed cells catabolize their own contents including long-lived proteins and cytoplasmic organelles to provide cellular energy and building blocks for biosynthesis, is one of the cellular mechanisms for maintenance of homeostasis. In addition to its basic role in the turnover of proteins and organelles, autophagy has multiple physiological and pathophysiological functions.1, 2, 3 Autophagy takes place when cells encounter environmental stressors, such as nutrient starvation, change in cell volume, oxidative stress, accumulation of misfolded protein, hormonal signaling, irradiation, xenobiotic treatment, and pathogen infection and participates in tissue remodeling during development and differentiation and elimination of excessive or injured organelles and molecules.1, 2, 3, 4, 5 Autophagy dysfunction is associated with several pathological conditions, such as hereditary myopathies,6 retinal degeneration,7 tumorigenesis,8, 9, 10 and neurodegenerative disorders.11
While the catabolic advantage of autophagy may be critical for organism survival during various stress conditions, autophagy may inhibit malignant transformation and tumor growth.12 A correlation between reduced autophagy and cancer development has been documented for nearly two decades.13 When baseline levels were compared, the amount of proteolysis or autophagic degradation in cancer cells was less than that of their normal counterparts.14 Studies have shown that tamoxifen, temozolomide, rapamycin, PI-3K/PKB inhibitors, and HDAC inhibitors as well as irradiation all induce autophagic cell death in malignant cells.14These findings suggest that induction of autophagic cell death may provide an alternative therapeutic mechanism, in particular in apoptosis-resistant tumor cells.
We recently discovered a family of plant triterpene electrophiles, avicins (Supplementary Figure 1), selectively inhibit growth in a wide variety of tumor cells.15, 16, 17 Avicins activate the intrinsic caspase pathway to induce apoptosis by direct perturbation of mitochondria and by downregulation of Bcl2 family prosurvival, antiapoptotic proteins that function downstream of cytochrome c release from mitochondria.15, 16, 17, 18 In addition to the cytotoxic properties, avicins also demonstrate cytoprotective effects in non-transformed cells by the activation of Nrf-2 and its downstream targets.19 These studies indicate that avicins are multi-functional compounds, which may play a role in the maintenance of cellular homeostasis.
One of the most significant roles that avicins play in tumor suppression is in disrupting mitochondrial metabolism, causing intracellular energy crisis. Avicins increase mitochondrial outer membrane permeability, and decrease oxygen consumption.15, 19 Since autophagy is a common response to nutrient starvation and mitochondrial dysfunction,20, 21 we reasoned that avicins may induce autophagy and/or autophagic cell death by interfering with cellular bioenergetics. We demonstrate that avicins can trigger caspase-independent autophagic cell death by the regulation of AMP-activated protein kinase (AMPK)-tuberous sclerosis complex 2 (TSC2)-mammalian target of rapamycin (mTOR) pathway, implicating the potential use of avicins in apoptosis-resistant cancers. Thus, avicins could serve as an important new chemical template for the treatment and/or prevention of cancer.
Results
Avicin D induces non-apoptotic cell death
To test whether avicins can induce non-apoptotic cell death, human osteosarcoma cells (U2OS) were treated with benzyloxycarbonylvalyl-alanyl–aspartic acid (O-methyl)–fluoro-methylketone (zVAD-fmk), a pan-apoptotic inhibitor, before incubation with avicin D (one of the avicin isoforms). Consistent with previous reports, avicin D induced the activation of caspase-7 and -8 (Figure 1a and b). The activation was reduced significantly by pretreatment with zVAD–fmk (Figure 1a and b). However, the cells treated with avicin D in the presence of zVAD–fmk for up to 48 h still underwent cell death (Figure 1c and d) although the extent of cell death in zVAD–fmk-treated cells was less than that observed in the cells treated with avicin D alone (Figure 1c). After the treatment of avicin D, the cells became rounded, irregular and then ballooned (data not shown). Taken together, these data suggest that avicin D can induce non-apoptotic cell death.
Avicin D induces non-apoptotic cell death. U2OS cells were pretreated with zVAD (50 μM) for 2 h, followed by avicin D (2 μg/ml) for 48 h. (a) Cleavage of caspase-7 induced by avicin D and inhibition of the cleavage were analyzed by western blotting. (b) Caspase-8 activity was measured by using caspase-8 luminescence assay. Data are shown as mean±S.D. (n=3). (c and d) Cell death (c) and cell viability (d) were measured as described in the methods. Data are shown as mean±S.D. (n=5)
Avicin D induces autophagy
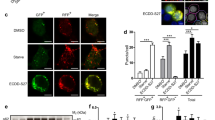
Since autophagic cell death is an important type of non-apoptotic cell death, we asked whether avicin D-induced non-apoptotic cell death could result from autophagy. To visualize autophagy, U2OS/GFP-light-chain 3 (LC3), a stable cell line expressing green fluorescent protein (GFP)-tagged LC3 was established. During the autophagic process, LC3 is concentrated in autophagosomes forming cytosolic punctate fluorescence, which serves as an indicator of autophagy.22, 23 As shown in Figure 2a a redistribution of GFP-LC3 from diffuse cytoplasmic location in untreated cells to discrete vesicular structures (punctate fluorescence) upon avicin D treatment was observed. The punctate GFP-LC3 fluorescence was induced as early as 2 h after avicin D treatment (Supplementary Figure 2). The number of the cells with punctate GFP-LC3 fluorescence increased in a time-dependent manner following the addition of avicin D (Figure 2b). zVAD–fmk itself induced very few punctate GFP-LC3 fluorescent vesicles (Figure 2a and b). This redistribution was confirmed biochemically by western blot analysis of LC3. Intracellular LC3 of avicin D-treated cells underwent a conversion from the LC3-I isoform to the LC3-II isoform, which is specific for autophagosomes (Figure 2c).4
Avicin D induces autophagy. U2OS/GFP-LC3 cells and U2OS cells carrying empty vector were pretreated with zVAD (50 μM) for 2 h, followed by avicin D (2 μg/ml) from 0 to 48 h. (a) Punctate GFP-LC3 fluorescence in U2OS/GFP-LC3 stable cells. Arrows indicate the autolysosomes/autophagosomes. (b) The percentage of cells with punctate GFP-LC3 fluorescence was calculated relative to all GFP-positive cells at the indicated time points. Data are shown as mean±S.D. (n=6). (c) Expression of GFP-LC3-I and II in U2OS/GFP-LC3 stable cells treated with/without avicin D and/or zVAD. (d) Electron micrograph of U2OS/GFP-LC3 cells treated with zVAD and avicin D for 48 h. A large number of autolysosomes/autophagosomes were observed, but the nuclei were unaffected (III). Representative autophagosomes that had double-layer membranes are also shown (II and IV). Black arrows indicated the autolysosomes/autophagosomes. Blue arrows indicate the membranes of autolysosomes/autophagosomes. The scale bars represent 10 μm in I and III and 0.5 μm in II and IV. N, nucleus
Electron microscopy of avicin D-treated U2OS cells showed the formation of double- or multi-membrane-bound structures containing recognizable cellular organelles and high electron-density substance characteristic of autophagosomes and autolysosomes (Figure 2d, Supplementary Figure 3). In contrast, the majority of the nuclei remained to normal appearance in avicin D- and zVAD–fmk-treated cells, and neither chromatin condensation nor nuclear fragmentation was observed, confirming the absence of apoptotic cell death (Figure 2d).
To determine whether avicin D-induced autophagy is generalizable, we transfected GFP-LC3 into several other cell lines, MDA-MB-231, T47D (human breast cancer cell lines), A549 (human alveolar epithelial cancer cell line), SKOV3 (ovarian cancer cell line), and PC3 (prostate cancer cell line) and exposed these cells to 50 μM zVAD–fmk and 2 μg/ml avicin D for 24 h. Consistent with the findings in U2OS cells, the diffuse GFP fluorescence was observed in control and zVAD–fmk-treated alone cells (Supplementary Figure 4). Punctate GFP-LC3 staining was induced in all cell lines following exposure to avicin D in the presence or absence of zVAD–fmk (Supplementary Figure 4). Avicin D treatment also led to 35–65% increase in cell death in these cells (Supplementary Figure 5). Taken together, our data indicate that avicin D induces autophagy in human cancer cells derived from a range of tissue origins.
Suppression of autophagy abrogates avicin D-induced non-apoptotic cell death
To assess the role of autophagy in avicin D-induced non-apoptotic cell death, the effects of chloroquine (CQ), a lysosomotropic agent that inhibits vesicle acidification and autophagy, were tested. Pretreatment with CQ for 6 h completely inhibited cell rounding and detachment of avicin D-treated cells (data not shown). These cells also showed a significant reduction in cell death (Figure 3a), and a marked improvement in cell viability relative to cells treated with avicin D alone (Figure 3b), suggesting that avicin D-induced cell death is dependent on lysosome.
Inhibition of avicin D-induced cell death by autophagy inhibitors. (a and b) U2OS/GFP-LC3 as well as control cells were pretreated with 10 μM CQ for 6 h. zVAD (50 μM) and avicin D (2 μg/ml) were then added as described in Figure 2. The cells were collected for the analysis post 48 h treatment. Cell death (a) and cell viability (b) were measured as described in the Materials and Methods section. Data are shown as mean±S.D. (n=4). (c–f) U2OS/GFP-LC3 cells were mock treated or treated with siRNA of Atg5 and Atg7 or non-targeting siRNA for 24 h. The cells were then cultured in the presence or absence of avicin D (2 μg/ml) and zVAD (50 μM) for another 48 h. (c) Punctate GFP-LC3 fluorescence in U2OS/GFP-LC3 cells treated with avicin D in the presence or absence of Atg5 and Atg7 siRNA. (d) Cell viability (upper panel) and cell death (bottom panel) in U2OS/GFP-LC3 cells treated with Atg5 and Atg7 siRNA in the presence or absence of zVAD and avicin D (mean±S.D., n=4). (e) Expression of Atg5 and GFP-LC3 in Atg5 siRNA cells. (f) Expression of Atg7 and GFP-LC3 in Atg7 siRNA cells. (g) Bax−/−Bak−/− cells pretreated with 10 μM CQ for 6 h, followed by avicin D (2 μg/ml) for 48 h, were analyzed for cell viability (left panel) and cell death (right panel) (mean±S.D., n=4)
To further ascertain the involvement of autophagic process in avicin D-induced non-apoptotic cell death, the effects of autophagy-related gene-5 (Atg5) and Atg7 depletion by gene silencing were investigated. Atg5 is essential for the generation of autophagosomes by forming conjugates with Atg12. Atg7 is an ubiquitin-activating enzyme E1-like protein, mediating a critical step in Atg12–Atg5 and Atg8–phosphatidylethanolamine conjugation. Small interfering RNA (RNAi) targeting Atg5 and Atg7 significantly reduced endogenous expression of Atg5 and Atg7 and reversed the effect of avicin D on autophagosome formation and cell viability (Figure 3c–f). During avicin D treatment a large fraction of the Atg5- and Atg7-silenced cells appeared healthy and the formation of autophagosomes was markedly suppressed (Figure 3c). The majority of the cells remained viable irrespective of the addition of avicin D (Figure 3d, upper panel) whereas the cell death was substantially reduced (Figure 3d, bottom panel). Taken together, these data strongly suggest that avicin D-induced non-apoptotic cell death is associated with autophagic process.
Avicin D induces autophagic cell death in Bax−/−Bak−/− cells
To further demonstrate that avicin D induces apoptosis-independent cell death, we used an immortalized interleukin-3 (IL-3)-dependent cell line derived from bone marrow cells of Bax−/−Bak−/− mice. These cells failed to undergo apoptosis in response to various apoptotic stimuli including avicin D (data not shown).4 However, cell death was markedly increased and cell viability was reduced after treatment with avicin D, and much of the effect was abrogated by the addition of CQ (Figure 3g). These results provide further evidence that avicin D can induce non-apoptotic cell death in apoptosis-resistant cells.
Avicin D-induced cell energy deficiency contributes to autophagy
To determine whether avicin D directly affects the expression of autophagy-associated genes, we first examined the expression of Beclin 1. As shown in Figure 4a, U2OS/GFP-LC3 cells treated with avicin D and/or zVAD–fmk, showed no accumulation of Beclin 1. Similarly, no changes were seen in the levels of Atg5 and Atg7 proteins (Figure 3e and f).
Avicin D-induced cell energy deficiency contributes to autophagy. (a) Expression of beclin 1 and phospho-Akt (S473) in U2OS/GFP-LC3 cells treated with avicin D (2 μg/ml) and/or zVAD (50 μg/ml) for 48 h. (b) Punctate GFP-LC3 fluorescence in GFP-LC3 and PKBDD expressing U2OS cells that were treated with avicin D for 48 h. (c) Expression of Akt (PKBDD) in the transfected cells. (d) The percentage of cells with punctate GFP-LC3 fluorescence was calculated relative to all GFP-positive cells in co-transfected cells treated with avicin D. Data are shown as mean±S.D. (n=3). (e) Localization of 4F2hc in U2OS cells treated with 2 μg/ml avicin D from 0 to 12 h, or U2OS cells cultured in the medium without amino acids (aa) for 20 min. (f) Amino acid uptake in U2OS cells treated with 2 μg/ml avicin D from 0 to 24 h. Data represent average of three independent experiments±S.D. (g) ATP levels of cells grown in 2 μg/ml avicin D for 0–72 h. Data represent average of three independent experiments±S.D.
Avicin D has been previously shown to inhibit the activation of Akt,16 a pivotal prosurvival and pro-oncogenic protein that is activated by serine and threonine phosphorylation.24 Akt is believed to contribute to the activation of mTOR, which is a crucial autophagy regulator,24, 25 either directly or indirectly through phosphorylation and suppression of TSC2. To evaluate the role of Akt in avicin-induced autophagy, we co-transfected Akt dominant active expression plasmid (PKBDD) with GFP-LC3. The number of cells with punctate GFP-LC3 fluorescence were comparable between PKBDD and vector-transfected cells after avicin D treatment (Figure 4b–d). Thus, constitutive activation of Akt did not block avicin D-mediated autophagy.
Amino acid deficiency or aberrant metabolism is one of the most common stimuli that induce autophagy. To address if impaired amino acid uptake contributed to avicin D-induced autophagy, we stained the cells with anti-4F2hc antibody. 4F2hc (type II glycosylated integral membrane protein 4F2 heavy chain) mediates neutral amino acid transport through its dimerizing with 4F2 light chain (4F2 lc). 4F2hc may also participate in intracellular amino acid trafficking.26 Immunofluorescence staining showed that avicin D did not affect the location of 4F2hc, which was chiefly membrane and cytoplasmic in cells cultured in amino acid-containing medium (Figure 4e). On the contrary, depletion of amino acids triggered the translocation of 4F2hc to the nucleus within 20 min (Figure 4e). To exclude the possibility that avicin D may affect amino acid transport via 4F2hc independent mechanisms, amino acid uptake assays were performed. Only marginal decrease in the amino acid uptake kinetics was detected in the presence of avicin D for up to 24 h (Figure 4f). These results indicate that avicin D-associated autophagic cell death was not due to the deregulation of amino acids.
We have demonstrated that avicins perturb mitochondrial function, and disrupt the integrity of mitochondria.15 Mitochondria provide the cell with energy, which is an important determinant for autophagy formation.20, 21 Thus, avicin D might induce autophagy by affecting cellular bioenergetics. To test this possibility, we monitored changes in cellular ATP levels in response to avicin D. U2OS cells treated with avicin D showed a significant time-dependent decrease in the levels of ATP (Figure 4g), thereby suggesting that cellular energy depletion could be responsible for avicin-induced autophagy. Therefore, we next determined whether the depletion of ATP production in avicin-treated cells could be reversed by pretreating cells with a cell-permeable form of pyruvate, methylpyruvate (MP), which can be oxidized in the tricarboxylic acid cycle to produce NADH that fuels the electron transport and ATP production.3, 4 MP restored ATP production in avicin D-treated cells to levels that were close to those observed in untreated cells (Supplementary Figure 6a). MP suppressed the avicin D-induced cell death (Supplementary Figure 6b) and increased the viability of avicin D-treated cells (Supplementary Figure 6c). The development of autophagosomes/autolysosomes was inhibited after addition of MP (Supplementary Figure 6d). Collectively, our data indicate that avicin D-induced disruption of cellular bioenergetics contributes to autophagy formation and mediates non-apoptotic cell death.
Avicin D induces autophagy via the activation of AMPK
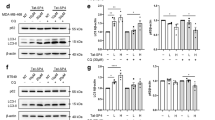
On the basis of previous finding that lowered levels of ATP might contribute to avicin D-mediated autophagy, we measured the phosphorylation of AMPK. AMPK is activated as intracellular AMP/ATP ratios rise.27, 28, 29 Avicin D treatment caused a significant increase of AMPK phosphorylation at Thr-172 (Figure 5a, Supplementary Figure 7). Phospho-AMPKThr-172 phosphorylates and activates TSC2, further inhibiting the activation of their downstream targets, such as mTOR and S6.27, 28 Phosphorylation of mTOR and S6 proteins was substantially decreased in avicin D-treated cells (Figure 5a). To address whether AMPK activation plays an essential role in avicin D-induced autophagy formation, we determined the ability of avicin D to induce autophagy in AMPK suppressed cells by (a) treating the cells with an AMPK inhibitor (compound C)30 and (b) transfecting the cells with dominant-negative AMPK.31 Compound C inhibited avicin-induced phosphorylation of AMPK and cell autophagy (Figure 5a and b), attenuated avicin D-triggered cell death (Figure 5c), and improved the cell viability of avicin D-treated cells (Figure 5d). To further confirm the role of AMPK in avicin D-induced autophagy, we constructed a stable cell line, U2OS/AMPK-DN, which express dominant-negative AMPK. The degree of autophagy in U2OS/AMPK-DN cells was about 50% of that in parental U2OS cells treated with avicin D (data not shown). Cell death induced by avicin D was significantly inhibited in U2OS/AMPK-DN cells (Figure 5e), and the cell viability was increased after avicin D treatment in these cells (Figure 5f). These data indicate that AMPK or its downstream targets are important mediators of avicin D-induced autophagic cell death.
Avicin D induces autophagy via the activation of AMPK. (a) Expression of phospho-AMPK (T172), AMPK, phospho-mTOR (S2448), and phospho-S6 (S235/236) in U2OS/GFP-LC3 cells treated with 2 μg/ml avicin D and/or 10 μM compound C for 24 h or in U2OS/AMPK-DN cells treated with 2 μg/ml avicin D for 24 h. (b) Punctate GFP-LC3 fluorescence in U2OS/GFP-LC3 cells treated with avicin D in the presence or absence of compound C. U2OS/GFP-LC3 cells were pretreated with 10 μM compound C for 8 h followed by 2 μg/ml avicin D treatment for 48 h. (c and d) Cell death (c) and cell viability (d) in avicin D and/or compound C (CC)-treated cells. Data are shown as mean±S.D. (n=3). (e and f) U2OS and U2OS/AMPK-DN cells were treated with 0, 1, 2, and 4 μg/ml of avicin D for 48 h. After the treatment, the cell death (e) and cell viability (f) were analyzed. Data represent average of three independent experiments±S.D.
Knock down of TSC2 suppresses avicin D-induced autophagic cell death
To elucidate the role of AMPK-TSC2-mTOR pathway in avicin D-induced autophagic cell death, the effects of TSC2 depletion by gene silencing were investigated. Small RNAi targeting TSC2 significantly reduced endogenous expression of TSC2 and reversed the inhibitory effect of avicin D on mTOR and S6 phosphorylation (Figure 6a). In contrast, as expected, AMPK and its upstream kinase, LKB1, were not affected by TSC2 RNAi (Figure 6a). During avicin D treatment a large fraction of the TSC2-silenced cells appeared healthy and the formation of autophagosomes was markedly suppressed (Figure 6b and c). The majority of the cells remained viable irrespective of the addition of avicin D (Figure 6d and e). In contrast, TSC2 silencing did not block the ability of rapamycin, an inhibitor of mTOR to induce autophagy (Figure 6b–e). Taken together, these data strongly support the hypothesis that AMPK-TSC2-mTOR pathway activation plays an important role in avicin D-induced autophagic cell death.
Avicin D regulates AMPK-TSC2-mTOR pathway. (a) Expression of phospho-AMPK (T172) and its target genes in TSC2 siRNA cells. (b) Punctate GFP-LC3 fluorescence in U2OS/GFP-LC3 cells treated with avicin D in the presence or absence of TSC2 siRNA. Twenty-four hours post-transfection, cells were treated with 2 μg/ml avicin D or 20 nM rapamycin and examined after another 48 h. (c) The percentage of cells with punctate GFP-LC3 fluorescence to all GFP-positive cells treated with TSC2 siRNA and avicin D or rapamycin (Rap.). Data represent average of three independent experiments±S.D. (d and e) Cell viability (d) and cell death (e) in U2OS/GFP-LC3 cells treated with TSC2 siRNA and avicin D or rapamycin (Rap). Data represent average of three independent experiments±S.D. (f) Role of avicins in autophagy. Schematic diagram depicting mechanism of action of avicins to induce autophagy. Avicins increase the AMP/ATP ratio thereby regulating the AMPK-TSC2-mTOR pathway and enhance cell autophagy
Discussion
The emerging recognition of the intersection of metabolic stress, inflammation and cancer has stimulated a search for compounds that can re-establish metabolic homeostasis. In the present study, we demonstrate that avicin D induces cell autophagy, a pathway by which energy-demanding cells can be eliminated, thereby reducing organical stress.32, 33 We demonstrated that avicin D induced a caspase-independent autophagic cell death, which could be suppressed by CQ and knockdown of Atg5 and Atg7. We showed evidence that avicin D decreased ATP levels resulting in the activation of AMPK, one of the key mediators of autophagy presumably through altered ATP/AMP ratios.31, 34 Furthermore, we showed that inhibition of AMPK activity either by compound C or by a dominant-negative form of AMPK significantly reduced avicin D-induced autophagic cell death. Knockdown of TSC2, a downstream target of AMPK, also abrogated the effect of avicin D on autophagy. In addition, application of avicin D to mice exposed to DMBA significantly decreased the expression of phospho-mTOR and increased autophagosome formation (data not shown). These observations clearly demonstrate that avicins can induce autophagic cell death by regulation of AMPK-TSC2-mTOR pathway and suggest that this may contribute to antitumor activity (Figure 6f).
Of note, neither CQ nor Atg5 and Atg7 depletion can completely abrogate the effect of avicin D on cell death, consistent with the ability of avicins to induce apoptotic cell death as we reported previously.15, 16, 17, 18 Interestingly, less cell death was observed in CQ-treated or Atg depleted U2OS cells than in cells treated with compound C, although all blocked autophagy efficiently. Therefore, in contrast to the role of the AMPK pathway in mediating avicin D-induced autophagy, AMPK activation may also provide a protection against apoptosis.34
Autophagy is induced with metabolic energetic stress. In common species, for example, yeast, flies, plants, and worms, autophagy is primarily an adaptive survival response to food deprivation. Humans, too, have evolved molecular mechanism to maintain homeostasis during cellular metabolic stress. The initial sensor of cellular bioenergetic crisis is AMPK.34, 35 Its downstream target, mTOR integrates signals from nutrients as well as growth factors to control cell growth.25, 31 Thus, mTOR acts as a metabolic rheostat controlling protein synthesis during cellular stress. Autophagy mediating catabolism of intracellular contents can maintain cellular bioenergetics during metabolic stress. The evolutionary role of autophagy is to facilitate cell survival during energy-deprivation states. The emergence of a cytotoxic function, primarily for energy-consuming tumor cells, probably evolved layered on top of the survival function.
The role of autophagy in tumorigenesis and tumor suppression is not completely clear. Generally, autophagy degrades long-lived proteins and organelles as an adaptive response to nutrient deprivation and other forms of cellular stress. Therefore, macromolecules and damaged organelles can be re-cycled or removed.9, 11, 36 It is thought that autophagy decreases the mutation rate and suppresses oncogenesis by eliminating damaged organelles that produce genotoxic stresses such as free radicals. On the other hand, in well-developed solid tumors, cancer cells at the center of a mass are poorly vascularized, hypoxic, and likely under decreased extracelluar glucose, amino acid, and fatty acid levels.14 The induction of autophagy may allow the stressed cells to survive in the tumor microenvironment.10, 14, 37 However, if levels of autophagy are induced beyond a physiological range, the autophagy pathway can contribute to cell death.14 Consistent with this idea, many cancer cells might have lost the ability to undergo this form of non-apoptotic death as a growth advantage. Breast, ovarian, and prostate cancer cells frequently delete one allele of beclin 1, a mammalian homologue of yeast Atg6, which plays an important role in the induction of autophagy in response to starvation. Beclin 1+/− mice showed an increased incidence of bronchial carcinoma, hepatocellular carcinoma, and B-cell lymphomas.12, 36 Ectopic expression of beclin 1 and/or its binding protein UVRAG (UV-irradiation resistance-associated gene) induced caspase-independent autophagic cell death, further proving the role of autophagy in non-apoptotic programmed cell death.12, 36, 38
Most, if not all, radiation- and chemotherapy-resistant cancers have apoptotic defects. In particular, the mitochondria/cytochrome c pathway of apoptosis is frequently deregulated in human cancer tissues.14, 39 For example, Bax/Bak expression is severely attenuated in many malignancies, and antiapoptotic members of the Bcl-2 family are upregulated in a variety of human cancers.2, 39 Our work shows that even when Bax/Bak gene is deleted or caspases are inhibited, avicins can still trigger caspase-independent cell death via autophagy, suggesting potential therapeutic activity of avicins in apoptosis-resistant cancers. A recent report showed that Bcl-2 bound and interacted with Beclin 1, and inhibited Beclin1-induced cellular autophagy in response to nutrient deprivation or other specific autophagy stimuli,36 raising the possibility that Bcl-2 family members may function as oncogenes, not only by blocking apoptosis but also by blocking autophagy.36, 39 Importantly, avicin D treatment dramatically downregulates Bcl-2 expression (Our unpublished data), adding additional support to avicin-induced autophagy formation.
Taken together, these data, combined with our published reports15, 19 suggest that avicins can induce not only apoptotic cell death, but also autophagic programmed cell death by depletion of cell energy supply. Most importantly, our results show that even when proapoptotic genes are deleted or caspases are inhibited, avicins can still trigger cell death, implicating the potential therapeutic application of avicins in apoptosis-resistant cancers.
Materials and Methods
Antibodies and chemicals
Antibodies against caspase-7 and 4F2hc were purchased from BD Pharmingen (San Diego, CA, USA). Antibodies against Akt, Akt-S473, S6, S6-S235/236, mTOR, mTOR-S2448, AMPK, AMPK-T172, and TSC2 were purchased from Cell Signaling (Beverly, MA, USA). Anti-LKB1 was from Abcam Inc. (Cambridge, MA). Anti-Atg5 antibody was purchased from ProteinTech Group Inc. (Chicago, IL, USA). Anti-Atg7 antibody was purchased from Abgent Inc. (San Diego, CA, USA). Anti-beclin 1 polyclonal antibody was purchased from Novus Biologicals Inc. (Littleton, CO, USA). Anti-GFP and anti-α-tubulin monoclonal antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Sigma-Aldrich (St. Louis, MO, USA), respectively. MP and CQ were purchased from Sigma-Aldrich. zVAD-fmk was purchased from Biomol International (Plymouth Meeting, PA, USA). Avicin D and avicin G were purified from Acacia victoriae root extracts as described previously.18 Compound C was provided by Merck & Co. Inc. (Rahway, NJ, USA).30 Rapamycin was purchased from Cell Signaling.
Cell lines and DNA transfection
A549, SKOV3, PC3, and U2OS were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) in a humidified incubator containing 5% CO2 at 37°C. MDA-MB-231 and T47D human breast cancer cell lines were grown in medium RPMI-1640 with 10% FBS. Immortalized IL-3-dependent mouse Bax−/−Bak−/− cells, kindly provided by Dr. Craig B Thompson, were cultured as described previously.4 To establish U2OS/GFP-LC3 and U2OS/AMPK-DN stable cell lines, proliferating U2OS cells were transfected with GFP-LC3 or AMPK-DN plasmid. Forty-eight hours post-transfection, positive stable clones were selected by growing the cells with G418 (800 μg/ml) for 2 weeks.
SiRNA
A pool of four siRNA duplexes targeting human TSC2, Atg5, Atg7, and a non-targeting siRNA pool were purchased from Dharmacon Inc. (Lafayette, CO, USA), and transfection was performed according to the manufacturers protocol.
Measurement of ATP
ATP levels were measured using Bioluminescence Assay Kit CLS II from Roche Scientific (Indianapolis, IN, USA), as per the manufacturers protocol.
Caspase-8 activity assay
Caspase-8 activity was measured by a caspase-8 assay kit from Promega Corp. (Madison, WI, USA) according to the manufacturers protocol.
Cell viability and cell death assay
Cell viability was measured by a CellTiter-Glo Luminescent Cell Viability Assay kit from Promega Corp., as per the manufacturer's protocol. Cell death was measured by Trypan blue (Sigma-Aldrich)-exclusion assay.
Amino acid uptake assays
Amino-acid uptake in U2OS cells was performed as described previously with minor modifications.40 Briefly, U2OS cells were grown to 80% confluence in six-well plates in DMEM supplemented with 10% FBS. Cells were treated with avicin D for 0–24 h. Thereafter, cells were washed two times with PBS followed by two washes with Krebs–Ringer buffer (KRB) and incubated in KRB for 30 min at 37°C. About 1 μCi of tritiated amino-acid mixture ([3H] amino acids, 1 mCi/ml : TRK 440, Amersham Biosciences) was added to each well and the uptake was performed for 5 min at 37°C. The uptake was stopped by washing the cells three times with ice-cold PBS. The cells were harvested under cold conditions, lysed in a buffer containing 0.5% NP-40 and the radioactivity incorporated was measured using Beckman Scintillation counter using CytoScint medium (ICN). The clear lysate was used to measure the protein content (Bio-Rad) to normalize the amino-acid uptake to the protein content.
Immunofluorescence and fluorescence microscope
The cells were grown in six-well plates with cover slides and fixed in cold 4% neutral paraformaldehyde in PBS for 30 min on ice, washed in PBS, permeabilized in a 1% Triton X-100/0.5% NP-40/PBS, and blocked in 1% bovine serum albumin in PBS. Incubation with a primary antibody was carried out for 2 h at room temperature. Incubation with a secondary antibody was carried out for 1 h at room temperature followed by staining of DNA with 4,6-diamidino-2-phenylindole for 5–10 min. Slides were mounted with Vectashield antifade medium (Vector Laboratories) after three washes with washing buffer and examined under NIKON ECLIPSE TE2000-E fluorescence microscope. The location and distribution of GFP-LC3 staining was examined directly as described previously22, 23 using a NIKON ECLIPSE TE2000-E fluorescence microscope.
Immunoblotting
Cell lysis and immunoblotting were performed as described previously.17 A total of 50 μg protein was used for the immunoblotting, unless otherwise indicated. α-Tubulin was used for the loading control.
Electron microscopy
Cells were fixed with 2% paraformaldehyde/2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4), followed by 1% OsO4. After dehydration, thin sections were stained with uranyl acetate and lead citrate for observation under a JEM 100 CX electron microscope.4
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- Atg:
-
autophagy-related gene
- CQ:
-
chloroquine
- LC3:
-
light-chain 3
- MP:
-
methylpyruvate
- mTOR:
-
mammalian target of rapamycin
- TSC2:
-
tuberous sclerosis complex 2
- zVAD–fmk:
-
benzyloxycarbonylvalyl-alanyl–aspartic acid (O-methyl)–fluoro-methylketone
References
Levine B, Klionsky DJ . Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 2004; 6: 463–477.
Criollo A, Maiuri MC, Tasdemir E, Vitale I, Fiebig AA, Andrews D et al. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ 2007; 14: 1029–1039.
Qu X, Zou Z, Sun Q, Luby-Phelps K, Cheng P, Hogan RN et al. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell 2007; 128: 931–946.
Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 2005; 120: 237–248.
Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V . Autophagy is a defense mechanism inhibiting BCG and mycobacterium tuberculosis survival in infected macrophages. Cell 2004; 119: 753–766.
Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lüllmann-Rauch R et al. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 2000; 406: 902–906.
Shintani T, Klionsky DJ . Autophagy in health and disease: a double-edged sword. Science 2004; 30: 990–995.
Alva AS, Gultekin SH, Baehrecke EH . Autophagy in human tumors: cell survival or death? Cell Death Differ 2004; 11: 1046–1048.
Kroemer G, Jaattela M . Lysosomes and autophagy in cell death control. Nat Rev Cancer 2005; 5: 886–897.
Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB et al. Role of BCL-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nature Cell Biol 2004; 6: 1221–1228.
Komatsu M, Waguri S, Chiba T, Murata S, Iwata JI, Tanida I et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006; 441: 880–884.
Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999; 402: 672–676.
Gozuacik D, Kimchi A . Autophagy as a cell death and tumor suppressor mechanism. Oncogene 2004; 23: 2891–2906.
Kondo Y, Kanzawa T, Sawaya R, Kondo S . The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 2005; 5: 726–734.
Haridas V, Higuchi M, Jayatilake GS, Bailey D, Mujoo K, Blake ME et al. Avicins: triterpenoid saponins from Acacia victoriae (Bentham) induce apoptosis by mitochondrial perturbation. Proc Natl Acad Sci USA 2001; 98: 5821–5826.
Mujoo K, Haridas V, Hoffmann JJ, Wachter GA, Hutter LK, Lu Y et al. Triterpenoid saponins from Acacia victoriae (Bentham) decrease tumor cell proliferation and induce apoptosis. Cancer Res 2001; 61: 5486–5490.
Gaikwad A, Poblenz A, Haridas V, Zhang C, Duvic M, Gutterman JU . Triterpenoid electrophiles (avicins) suppress heat shock protein-70 and x-linked inhibitor of apoptosis proteins in malignant cells by activation of ubiquitin machinery: implications for proapoptotic activity. Clin Cancer Res 2005; 11: 1953–1962.
Haridas V, Arntzen CJ, Gutterman JU . Avicins, a family of triterpenoid saponins from Acacia victoriae (Bentham), inhibit activation of nuclear factor-kappaB by inhibiting both its nuclear localization and ability to bind DNA. Proc Natl Acad Sci USA 2001; 98: 11557–11562.
Haridas V, Hanausek M, Nishimura G, Soehnge H, Gaikwad A, Narog M et al. Triterpenoid electrophiles (avicins) activate the innate stress response by redox regulation of a gene battery. J Clin Invest 2004; 113: 65–73.
Jin S . Autophagy, mitochondrial quality control, and oncogenesis. Autophagy 2006; 2: 80–84.
Priault M, Salin B, Schaeffer J, Vallette FM, di Rago JP, Martinou JC . Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ 2005; 12: 1613–1621.
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T et al. LC3, a mammalian homologue of yeast APG8P, is localized in autophagosome membranes after processing. EMBO J 2000; 19: 5720–5728.
Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y . In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 2004; 15: 1101–1111.
Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell 2003; 11: 1457–1466.
Wullschleger S, Loewith R, Hall MN . TOR signaling in growth and metabolism. Cell 2006; 124: 471–484.
Nakamura E, Sato M, Yang H, Miyagawa F, Harasaki M, Tomita K et al. 4F2 (CD98) heavy chain is associated covalently with an amino acid transporter and controls intracellular trafficking and membrane topology of 4F2 heterodimer. J Biol Chem 1999; 274: 3009–3016.
Codogno P, Meijer AJ . Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 2005; 12: 1509–1518.
Kahn BB, Alquier T, Carling D, Hardie DG . AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005; 1: 15–25.
Jaleel M, Villa F, Deak M, Toth R, Prescott AR, Van Aalten DM et al. The ubiquitin-associated domain of AMPK-related kinases regulates conformation and LKB1-mediated phosphorylation and activation. Biochem J 2006; 394: 545–555.
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108: 1167–1174.
Inoki K, Zhu T, Guan KL . TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003; 115: 577–590.
Baehrecke EH . Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol 2005; 6: 505–510.
Bursch W . The autophagosomal–lysosomal compartment in programmed cell death. Cell Death Differ 2001; 8: 569–581.
Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 2007; 9: 218–224.
Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005; 310: 1642–1646.
Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N et al. Bcl-2 antiapoptotic proteins inhibit beclin 1-dependent autophagy. Cell 2005; 122: 927–939.
Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 2005; 25: 1025–1040.
Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BN et al. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol 2006; 8: 688–699.
Yuan J . Divergence from a dedicated cellular suicide mechanism: exploring the evolution of cell death. Mol Cell 2006; 23: 1–12.
Wasa M, Bode BP, Abcouwer SF, Collins CL, Tanabe KK, Souba WW . Glutamine as a regulator of DNA and protein biosynthesis in human solid tumor cell lines. Ann Surg 1996; 224: 189–197.
Acknowledgements
We thank Dr. Craig B Thompson for providing Bax−/− Bak−/− cells, Dr. Kun-Liang Guan for providing AMPK-DN plasmid, Dr. Gaochao Zhou from Merck Research Laboratories for providing compound C. We thank Dr. YL Lu for her helpful discussion. We thank Mr. K Dunner, Jr. and Ms. Shuangxing Yu for the expert technical assistance. This work was supported by the Clayton Foundation for Research, the Biomedical Research Foundation (to JUG), Developmental Research Awards from National Institutes of Health SPORE (P50-CA83639) (to Z.X.X), and MD Anderson Cancer Center Core Grant CA16672 from the National Cancer Institute, which supported the core facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by H-U Simon
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Supplementary information
Rights and permissions
About this article
Cite this article
Xu, ZX., Liang, J., Haridas, V. et al. A plant triterpenoid, avicin D, induces autophagy by activation of AMP-activated protein kinase. Cell Death Differ 14, 1948–1957 (2007). https://doi.org/10.1038/sj.cdd.4402207
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4402207
Keywords
This article is cited by
-
IL-33/ST2 antagonizes STING signal transduction via autophagy in response to acetaminophen-mediated toxicological immunity
Cell Communication and Signaling (2023)
-
Blockage of O-linked GlcNAcylation induces AMPK-dependent autophagy in bladder cancer cells
Cellular & Molecular Biology Letters (2020)
-
AMPK contributes to autophagosome maturation and lysosomal fusion
Scientific Reports (2018)
-
Piperlongumine and p53-reactivator APR-246 selectively induce cell death in HNSCC by targeting GSTP1
Oncogene (2018)
-
A Fucus vesiculosus extract inhibits estrogen receptor activation and induces cell death in female cancer cell lines
BMC Complementary and Alternative Medicine (2016)