Abstract
Aim:
To compare serum paraoxonase/arylesterase (PON-aryl) activities in phenylketonuric (PKU) patients with high and low phenylalanine (Phe) blood concentration.
Patients and methods:
Seventeen poorly controlled PKU children (off diet) underwent clinical and laboratory examinations before and after 30 days adhering to their special diet (on diet), whereas controls (N=24) were examined once. Lipid, lipoprotein levels and paraoxonase (PON 1) activities were measured with the Bayer Advia 1650 Clinical Chemistry System. Apolipoprotein AI (Apo AI) levels were determined by the Dade Behring BN ProSpec nephelometer, whereas total antioxidant capacity (TAC), PON-aryl and Phe levels were measured spectrophotometrically.
Results:
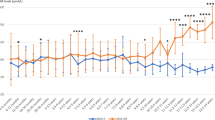
Phe significantly differed among the groups. Lipids and lipoproteins, except high-density-lipoprotein-cholesterol (HDL-C) and Apo AI, were higher when off diet than those on diet. HDL-C and Apo AI were similar in patients and controls. TAC (0.99±0.19 mmol/l) was significantly lower when the patients were off diet than when they adhered to diet and controls (1.71±0.20 and 1.81±0.20 mmol/l P<0.001 respectively). PON 1 and PON-aryl activities (68±2 U/min/ml, 88±26 KU (min/ml) in children with high Phe were reduced as compared with those with low blood Phe levels (152±41 U/min/ml, 107±23 KU/min/ml P<0.001) and controls (146±43 U/min/ml, 109±41 KU/min/ml P<0.001). The enzyme activities positively correlated with HDL-C and Apo AI when PKU patients were on diet and controls as well as with TAC in all the groups, whereas negatively correlated with Phe levels.
Conclusions:
PON-aryl activities are strongly related to the dietary control of PKU patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Aviram M, Billecke S, Sorenson R, Bisgaier C, Newton R, Rosental M et al. (1998). Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities. Arterioscler Thromb Vasc Biol 18, 1617–1624.
Aviram M, Hardak E, Vaya J, Mahmood S, Milo S, Hoffman A et al. (2000). Human serum paraoxnase (PON 1) Q and R selectively decrease lipid peroxides in human coronary and carotid atherosclerosis lesions: PON1 esterase and peroxidase-like activities. Circulation 101, 2510–2517.
Aviram M, Rosental M, Billecke S, Erogul J, Sorenson R, Bisgaier CL et al. (1999). Human serum paraoxonase (PON 1) in inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic Biol Med 26, 892–904.
Berliner JA, Heinecke JW (1996). The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med 20, 707–727.
Burgard P, Rupp A, Schneider W, Bremer HF (1996). Intellectual development of the patients of the German Collaborative Study of children treated for phenylketonuria. Eur J Pediatr 155 (Supp 1), 33–38.
Burkitt MJA (2001). Critical overview of the chemistry of copper-dependent low density lipoprotein oxidation: roles of lipid hydroperoxides, alpha-tocopherol, thiols and ceruloplasmin. Arch Biochem Biophys 394, 117–135.
Charlton-Menys V, Liu Y, Durrington PN (2006). Semiautomated method for determination of serum paraoxonase activity using paraoxon as substrate. Clin Chem 52, 453–457.
Costa L, Vitalone A, Cole TB, Furlong CE (2005). Modulation of paraoxonase (PON1) activity. Biochem Pharmacol 69, 541–550.
Duy Nguyen S, Dai Eun Sok (2003). Oxidative inactivation of paraoxonase 1, an antioxidant protein and its effect on antioxidant action. Free Radic Res 37, 1319–1330.
Ferre N, Camps J, Cabre M, Paul A, Joven J (2001). Hepatic paraoxonase activity alterations and free radical production in rats with experimental cirrhosis. Metabolism 9, 997–2000.
Francis D (1989). Diet for Sick Children. Blackwell Scientific: Oxford. pp. 603–609.
Halliwell B, Gutterdge JMC (1989). Free radicals Biol Med 2nd ed. Oxford Clarendon Press, Oxford, UK. pp 110–119.
Jaound L, Milochevitch C, Khalli A (2003). PON 1 Paraoxonase Activity is reduced during HDL oxidation and is an indicator of HDL antioxidant capacity. Free Radic Res 37, 77–83.
Josse D, Lockridge O, Xie W, Bartels CF, Schopfer LM, Masson P (2001). The active site of human paraoxonase (PON1). J Appl Toxicol 21, S7–S11.
La Du BN (1996). Structural and functional diversity of paraoxonase. Nat Medi 2, 1186–1187.
Lipid Research clinics Program. Mannual of Laboratory Operation, (1974): National Institute of Health 1 US Depart of Health Education and Welfare Publication (NIA): Bethesda MD. pp 75–628.
Mackness MI, Mackness B, Durrington PA, Connelly PW, Hegele RA (1996). Paraoxonase: biochemistry, genetics and relationship lipoproteins. Curr Opin Lipidol 7, 69–76.
Marathe GK, Zimmerman GA, McIntyre TM (2003). PAF acetylhydrolase, and not paraoxonase-1, is the oxidized phospholipids hydrolase of high density lipoprotein particles. J Biol Chem 278, 3937–3947.
McCall MR, Tang JY, Bielicki JK, Forte TM (1995). Inhibition of lecithin-cholesterol acyltransferase and modification of HDL apolipoproteins by aldehydes. Arterioscler Thromb Vasc Biol 15, 1599–1606.
Miller MJ, Rice Evans C, Davies MJ, Copinathan V, Milner A (1993). A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Soc (Colh) 84, 407–412.
Paul AA, Southgate DA, Russel J (1980). The composition of food. HMSO: London.
Reaven PD, Witzturn JL (1996). Oxidized low density lipoproteins in atherogenesis. A Rev Nutr 16, 51–71.
Schulpis KH, Karakonstantakis T, Bartzeliotou A, Karikas GA, Papassotiriou I (2004). The association of serum lipids, lipoproteins and apolipoproteins with selected trace elements and minerals in phenylketonuric patients on diet. Clin Nutr 23, 401–407.
Schulpis KH, Scarpalezou A (1989). Triglycerides, cholesterol, HDL, LDL and VLDL cholesterol in serum of phenylketonuric children under dietary control. Clin Pediatr 28, 466–469.
Schulpis KH, Tsakiris S, Karikas GA, Moukas M, Behrakis P (2003). Effect of diet on plasma total antioxidant status in phenylketonuric patients. Eur J Clin Nutr 57, 383–387.
Scriver CR, Beaudet AL, Valle D (eds.) (2000). The Hyperphenylalaninaemias in the Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill: New York, NY. pp. 1667–1725.
Senti M, Tomas M, Fito M, Weinbrenner T, Covas M, Sala J et al. (2003). Antioxidant paraoxonase 1 activity in the metabolic syndrome. J Clin Endocrinol Metab 88, 5422–5426.
Strube M, Haenen GR, Van der Beta H, Bast A (1997). Pitfalls in a method for assessment of total antioxidant capacity. Free Radic Res 26, 515–521.
Tsakiris S, Krontiri T, Schulpis KH, Stavridis J (1998). Phenylalanine effect on rat brain acetylcholinesterase and Na+,K+,ATPase. Z Naturforschung Part C 83, 163–167.
Tsakiris S, Schulpis KH, Karikas GA (2002). Reduced acetylcholinesterase activity in erythrocyte membranes from patients with phenylketonuria. Clin Biochem 35, 615–619.
Vlachos GD, Bartzeliotou A, Schulpis KH, Partsinevelos GA, Lazaropoulou C, Papadima C et al. (2006). Maternal-neonatal serum paraoxonase 1 activity in relation to the mode of delivery. Clin Biochem 39, 923–928.
Watson AD, Berliner JA, Hama SY, La Du BN, Fauli KF, Fogelman AM et al. (1995). Protective effect of high density lipoprotein associated paraoxonase: inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest 96, 2882–2891.
Wendel U, Kappelkamus M, Hammel W, Saunder J (1990). A new approach to the newborn screening for hyperphenylalaninemias use l-phenylalanine dehydrogenase and mixrotiter plates. Clin Chim Acta 192, 165–170.
Acknowledgements
We are highly indebted to Mrs Anna Stamatis for her careful typing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schulpis, K., Bartzeliotou, A., Tsakiris, S. et al. Serum paraoxonase/arylesterase activities in phenylketonuric patients on diet. Eur J Clin Nutr 61, 803–808 (2007). https://doi.org/10.1038/sj.ejcn.1602591
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602591
Keywords
This article is cited by
-
Oxidative stress in phenylketonuria—evidence from human studies and animal models, and possible implications for redox signaling
Metabolic Brain Disease (2021)
-
Effect of Blood Phenylalanine Levels on Oxidative Stress in Classical Phenylketonuric Patients
Cellular and Molecular Neurobiology (2018)
-
Oxidative stress in Phenylketonuria: future directions
Journal of Inherited Metabolic Disease (2012)