Abstract
Objective:
To test the dose-response effect on low-density lipoprotein cholesterol (LDL-c) of plant sterols (PS) from different sources in a low-fat spread.
Methods:
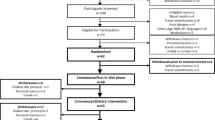
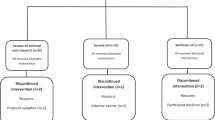
Dose responses of soybean oil (BO), tall oil (TO) and a mix of tall oil and rapeseed oil (TO/RP) as fatty acid esters were tested in a parallel design in free-living subjects recruited from the general community who had elevated cholesterol concentrations. Subjects received either control for 6 weeks or 1.6 g PS per day for 3 weeks, then 3.0 g/day for 3 weeks.
Results:
LDL-c was lowered significantly by consumption of 1.6 g/day of PS (−10.4%, range −7.3 to −11.4%). Increasing the dose to 3.0 g/day modestly reduced LDL-c concentrations further to −14.7%. TO, containing 78% sitosterol, produced an increase in serum sitosterol of 6.5 nmol/ml, while BO, containing only 27% campesterol, produced an increase in serum campesterol of 9.5 nmol/ml in 6 weeks. After PS withdrawal, serum sterols declined by 50% within 2 weeks.
Conclusion:
Different PS sources were equally effective in lowering serum LDL-c concentrations. The decrease in absolute concentrations of LDL-c was dependent on the baseline concentrations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
AbuMweis SS, Vanstone CA, Ebine N, Kassis A, Ausman LM, Jones PJH et al. (2006). Intake of a single morning dose of standard and novel plant sterol preparations for 4 weeks does not dramatically affect plasma lipid concentrations in humans. J Nutr 136, 1012–1016.
Albert MA, Danielson E, Rifai N, Ridker PM (2001). Effect of statin therapy on C-reactive protein levels - The Pravastatin Inflammation/CRP Evaluation (PRINCE): A randomized trial and cohort study. JAMA 286, 64–70.
Calpe-Berdiel L, Escola-Gil JC, Ribas V, Navarro-Sastre A, Garces-Garces J, Blanco-Vaca F (2005). Changes in intestinal and liver global gene expression in response to a phytosterol-enriched diet. Atherosclerosis 181, 75–85.
Clifton PM, Noakes M, Ross D, Fassoulakis A, Cehun M, Nestel P (2004). High dietary intake of phytosterol esters decreases carotenoids and increases plasma plant sterol levels with no additional cholesterol lowering. J Lipid Res 45, 1493–1499.
Devaraj S, Autret BC, Jialal I (2006). Reduced-calorie orange juice beverage with plant sterols lowers C-reactive protein concentrations and improves the lipid profile in human volunteers. Am J Clin Nutr 84, 756–761.
Gylling H, Miettinen TA (1995). The effect of cholesterol absorption inhibition on low-density lipoprotein cholesterol level. Atherosclerosis 117, 305–308.
Hallikainen MA, Sarkkinen ES, Uusitupa MI (2000). Plant stanol esters affect serum cholesterol concentrations of hypercholesterolemic men and women in a dose-dependent manner. J Nutr 130, 767–776.
Hallikainen M, Lyyra-Laitinen T, Laitinen T, Agren JJ, Pihlajamaki J, Rauramaa R et al. (2005). Endothelial function in hypercholesterolemic subjects: Effects of plant stanol and sterol esters. Atherosclerosis 188, 425–432.
Hendriks HFJ, Weststrate JA, van Vliet T, Meijer GW (1999). Spreads enriched with three different levels of vegetable oil sterols and the degree of cholesterol lowering in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur J Clin Nutr 53, 319–327.
Ho SS, Pal S (2004). Phytosterols decrease the secretion of atherogenic lipoproteins from HepG2 liver and Caco2 intestinal cells. Asia Pac J Clin Nutr 13 (Suppl), S68.
Jaye M (2003). LXR agonists for the treatment of atherosclerosis. Curr Opin Investig Drugs 4, 1053–1058.
Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P (2003). Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med 9, 213–219.
Kaneko E, Matsuda M, Yamada Y, Tachibana Y, Shimomura I, Makishima M (2003). Induction of Intestinal ATP-binding Cassette Transporters by a Phytosterol-derived Liver-X Receptor Agonist. J Biol Chem 278, 36091–36098.
Kesaniemi YA, Miettinen TA (1987). Cholesterol absorption efficiency regulates plasma-cholesterol level in the Finnish population. Eur J Clin Invest 17, 391–395.
Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R (2003). Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clinic Proc 78, 965–978.
Libby P, Ridker PM (2004). Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med 116, 9–16.
Matthan NR, Raeini-Sarjaz M, Lichtenstein AH, Ausman LM, Jones PJH (2000). Deuterium uptake and plasma cholesterol precursor levels correspond as methods for measurement of endogenous cholesterol synthesis in hypercholesterolemic women. Lipids 35, 1037–1044.
Mattson FH, Grundy SM, Crouse JR (1982). Optimizing the effect of plant sterols on cholesterol absorption in man. Am J Clin Nutr 35, 697–700.
Mattson FH, Volpenheim RA, Erickson BA (1977). Effect of plant sterol esters on the absorption of dietary cholesterol. J Nutr 107, 1139–1146.
Meijer GW, Bressers MAJJ, de Groot WA, Rudrum M (2003). Effects of structure and form on the ability of plant sterols to inhibit cholesterol absorption in hamsters. Lipids 38, 713–721.
Naumann E, Plat J, Mensink RP (2003). Changes in serum concentrations of noncholesterol sterols and lipoproteins in healthy subjects do not depend on the ratio of plant sterols to stanols in the diet. J Nutr 133, 2741–2747.
Nestel P, Cehun M, Pomeroy S, Abbey M, Weldon G (2001). Cholesterol-lowering effects of plant sterol esters and non- esterified stanols in margarine, butter and low-fat foods. Eur J Clin Nutr 55, 1084–1090.
Normén L, Dutta P, Lia A, Andersson H (2000). Soy sterol esters and beta-sitostanol ester as inhibitors of cholesterol absorption in human small bowel. Am J Clin Nutr 71, 908–913.
Ostlund RE, McGill JB, Zeng CM, Covey DF, Stearns J, Stenson WF et al. (2002). Gastrointestinal absorption and plasma kinetics of soy Delta(5)-phytosterols and phytostanols in humans. Am J Physiol Endocrinol Metab 282, E911–E916.
Plat J, Mensink RP (2002). Increased intestinal ABCA1 expression contributes to the decrease in cholesterol absorption after plant stanol consumption. FASEB J 16, 1248–1253.
Plosch T, Bloks VW, Terasawa Y, Berdy S, Siegler K, van der Sluijs F et al. (2004). Sitosterolemia in ABC-transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology 126, 290–300.
Ridker PM (2001). High-sensitivity C-reactive protein – potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 103, 1813–1818.
Salen G, Ahrens EH, Grundy SM (1970). Metabolism of β-Sitosterol in Man. J Clin Invest 49, 952–967.
Shepherd J, Bicker S, Lorimer AR, Packard CJ (1979). Receptor-mediated low-density lipoprotein catabolism in man. J Lipid Res 20, 999–1006.
Sierksma A, Weststrate JA, Meijer GW (1999). Spreads enriched with plant sterols, either esterified 4,4-dimethylsterols or free 4-desmethylsterols, and plasma total- and LDL-cholesterol concentrations. Br J Nutr 82, 273–282.
Sudhop T, Sahin Y, Lindenthal B, Hahn C, Luers C, Berthold HK et al. (2002). Comparison of the hepatic clearances of campesterol, sitosterol, and cholesterol in healthy subjects suggests that efflux transporters controlling intestinal sterol absorption also regulate biliary secretion. Gut 51, 860–863.
Trautwein EA, Duchateau GSMJE, Lin Y, Mel’nikov SM, Molhuizen HOF, Ntanios FY (2003). Proposed mechanisms of Cholesterol-lowering action of Plant Sterols. Eur J Lipid Sci Technol 105, 171–185.
Vissers MN, Zock PL, Meijer GW, Katan MB (2000). Effect of plant sterols from rice bran oil and triterpene alcohols from sheanut oil on serum lipoprotein concentrations in humans. Am J Clin Nutr 72, 1510–1515.
Weststrate JA, Meijer GW (1998). Plant sterol-enriched margarines and reduction of plasma total- and LDL-cholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur J Clin Nutr 52, 334–343.
Wolthers B, Walrecht H, van der Molen J, Nagel G, Van Doormaal J, Wijnandts P (1991). Use of determinations of 7-lathosterol (5 alpha-cholest-7-en-3 beta-ol) and other cholesterol precursors in serum in the study and treatment of disturbances of sterol metabolism, particularly cerebrotendinous xanthomatosis. J Lipid Res 32, 603–612.
Acknowledgements
The study was partially funded by Unilever. MI Beute and Y Veldhuizen from Unilever Research Vlaardingen are kindly acknowledged for the production of the different test products. H Steenbergen and H-G Janssen were instrumental in the sterol analysis of the sterol sources and final products. R Diks is kindly acknowledged for critical review and discussion of the results. R McArthur, K Bastiaan, C Sullivan, A McGuffin, J Weaver and C Keatch were responsible for the clinical study and J Keogh for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guarantor: PM Clifton.
Contributors: PMC and GSMJED designed the study; the intervention part was organized and monitored by PMC. PMC and MM coordinated the serum analysis. HCMvdK performed the data analysis and all authors participated in the manuscript writing, with the critical input of each during the raw data and statistics interpretation.
Rights and permissions
About this article
Cite this article
Clifton, P., Mano, M., Duchateau, G. et al. Dose-response effects of different plant sterol sources in fat spreads on serum lipids and C-reactive protein and on the kinetic behavior of serum plant sterols. Eur J Clin Nutr 62, 968–977 (2008). https://doi.org/10.1038/sj.ejcn.1602814
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602814
Keywords
This article is cited by
-
Phytosterol compositions of enriched products influence their cholesterol-lowering efficacy: a meta-analysis of randomized controlled trials
European Journal of Clinical Nutrition (2019)
-
The effect of plant sterols on serum triglyceride concentrations is dependent on baseline concentrations: a pooled analysis of 12 randomised controlled trials
European Journal of Nutrition (2013)
-
Dietary phytosterols and phytostanols decrease cholesterol levels but increase blood pressure in WKY inbred rats in the absence of salt-loading
Nutrition & Metabolism (2010)
-
Phytosterol Ester Constituents Affect Micellar Cholesterol Solubility in Model Bile
Lipids (2010)
-
The Metabolic Effects of Omega-3 Plant Sterol Esters in Mixed Hyperlipidemic Subjects
Cardiovascular Drugs and Therapy (2010)