Abstract
Purpose
To report our experience on the use of nonpreserved human amniotic membrane transplantation (AMT) in ocular surface reconstruction after excision of extensive ocular surface neoplasia (OSN).
Design
Prospective noncomparative interventional case series.
Participants
In all, 10 eyes of 10 consecutive patients with extensive OSN involving various areas of limbus, conjunctiva, and cornea (conjunctival carcinoma in situ, four eyes; squamous cell carcinoma, three eyes; malignant melanoma, two eyes; conjunctival-orbital lymphangioma, one eye) were included in this prospective noncomparative interventional case series. After excision of the neoplasia with 3–4 mm tumour-free margins, double freeze-thaw cryotherapy was applied to the margins of the remaining conjunctiva, and nonpreserved human amniotic membrane graft was sutured to the adjacent conjunctiva using 8/0 vicryl sutures and cornea using 10/0 nylon sutures, with the epithelial side facing up to cover the bare sclera and cornea. Postoperatively, topical corticosteroids were used for 3 months.
Results
After tumour excision and AMT, a satisfactory result with a wet, stable conjunctiva, and rapid and complete healing was observed in all eyes. Over a mean follow-up of 10.0 months (range, 6–27 months), all but one eye remained free of tumour recurrence. In one eye with conjunctival melanoma, there was a small recurrence, which was treated with excision and cryotherapy. Treatment complications were partial stem cell deficiency in two eyes and symblepharon formation in one eye. Immune graft rejection was not encountered.
Conclusion
Nonpreserved human AMT appears to be useful for reconstruction of ocular surface following excision of extensive OSN.
Similar content being viewed by others
Introduction
Ideal management of ocular surface neoplasia (OSN) consists of complete tumour excision, and physiologically and cosmetically acceptable reconstruction of the ocular surface without inflammation, vascularization or scarring.1 Surgical excision of extensive OSN results in wide tissue defects that cannot be closed primarily but require reconstruction with transpositional conjunctival flap, free conjunctival graft from the opposite eye, and/or oral mucosal grafts. However, in the case of conjunctival grafts, harvest of adequately sized conjunctival autografts may result in donor site morbidity, including scarring, secondary granulation tissue, symblepharon, restricted ocular/eyelid motility, and partial or total limbal stem cell deficiency.2, 3 Furthermore, the patients may refuse to have their healthy eye operated on for donor purposes. Thick mucosal (buccal or labial) grafts, on the other hand, are associated with an unsatisfactory cosmetic result, as they occupy large spaces in the fornix, may shrink with time, may mask regrowth of the underlying tumour, and invariably lead to a nonconjunctival epithelial morphology.2, 3
More recently, preserved amniotic membrane has been used for ocular surface reconstruction after excision of OSN. A number of authors have reported on the use of preserved amniotic membrane in the treatment of various conjunctival tumours, including intraepithelial neoplasia, squamous carcinoma, primary acquired melanosis, and melanoma with successful reconstruction of the ocular surface.4, 5, 6, 7 The purpose of this study is to evaluate the results of nonpreserved amniotic membrane transplantation in the reconstruction of ocular surface defects after excision of extensive OSN. As noted in the ‘Discussion’ section, nonpreserved amniotic membrane may provide certain advantages over preserved membrane. Extreme care must be taken in the processing of these tissues to prevent the transmission of infectious diseases.
Materials and methods
Between January 2001 and April 2004, 10 eyes of 10 patients with extensive OSN who underwent AMT were included in this prospective study. Data concerning age, sex, visual acuity, duration of symptoms, tumour size and location, and initial management before referral were collected.
Informed consent was obtained from all patients. The patients were tested for hepatitis B, hepatitis C, syphilis, and HIV the day before surgery. All surgeries were performed under retrobulbar block by two surgeons (KG and ÖÖU). Extensive ‘en bloc’ excisional biopsy of the lesion was performed with the ‘no touch’ technique, with care taken to resect 3–4 mm tumour-free margins. This was followed by cryotherapy to the conjunctival margins with double freeze-and-thaw technique. Cryotherapy was performed if a premalignant or malignant condition was suspected. During cryotherapy, the conjunctival edge was lifted up and the cryoprobe was placed under the resected edge of conjunctiva or onto the limbus. In each cycle, the cryoprobe was kept in contact with the tissue until an iceball extending 2 mm onto the conjunctiva and 0.5 mm onto the cornea was formed. A superficial keratectomy was performed if corneal infiltration was present. The tissues obtained from surgery were sent for pathological diagnosis. These procedures were followed by AMT.
Nonpreserved human amniotic membrane was used in all AMT procedures. The membrane was prepared in a fashion similar to a previously reported protocol.7, 8 The amniotic membrane was obtained from women undergoing elective cesarean section, after a 39-week pregnancy, who were seronegative for hepatitis B, hepatitis C, syphilis, and HIV the day before surgery. Under sterile conditions, a piece of membrane was separated from the placenta and placed in sterile saline solution. After being transported to our department, the amniotic membrane was dissected from the chorion with blunt dissection and copiously irrigated several times with saline solution containing 50 μg/ml penicillin, 50 μg/ml streptomycin, 100 μg/ml neomycin, and 2.5 μg/ml amphotericin B.7 The membrane was used immediately after processing as outlined above.
Amniotic membrane was trimmed to the appropriate size and placed over the denuded surface epithelial side up as a single layer. The membrane was sutured to the adjacent conjunctiva and episclera by interrupted 8/0 vicryl and to the cornea by interrupted 10/0 nylon sutures. In cases with fornicial involvement, the fornicial edge of the membrane was anchored with double-armed 6/0 vicryl sutures passing through the full thickness of the eyelid and tied over a bolster. If keratectomy was performed, patients were fitted postoperatively with a silicone hydrogel bandage contact lens (Focus Night and Day, CibaVision, Duluth, GA, USA). The lens was removed after complete re-epithelization of the cornea. If complete re-epithelization took more than a month, the contact lens was replaced at the end of 1 month.
All patients were given topical ofloxacin 0.3% four times daily (Exocin®, Allergan, Irvine, CA, USA) until epithelization occurred and topical prednisolone acetate 1% (Pred Forte®, Allergan, Irvine, CA, USA) four times daily for 4 weeks. Topical corticosteroids were tapered and discontinued in approximately 3 months. The nylon sutures were removed at postoperative week 2 or 3. Patients were instructed to use preservative-free tear substitutes (Refresh Tears®, Allergan, Irvine, CA, USA) every 2 h following surgery for 2 weeks, and four times daily thereafter for 6 months.
The patients were followed up at day 1, week 1, week 2, and at monthly intervals for the first 2 months and at 3-month intervals thereafter. At each follow-up examination, patients were questioned about any discomfort, visual acuity was measured, surface healing was evaluated by slit-lamp biomicroscopy and fluorescein staining, and possible complications such as inflammation, scarring, infection, immune reaction, and tumour recurrence were monitored. The final visual acuity was considered to be improved if the patient had <20/200 vision preoperatively and improved to ≥20/200 postoperatively, or if the preoperative vision was ≥20/200 and at least two lines of visual acuity improvement was seen at the last follow-up examination.
At postoperative month 6, the donor and the recipient patients were tested for HIV and hepatitis B and C.
Results
Table 1 lists patient demographics, preoperative and postoperative visual acuity in 10 eyes undergoing AMT. The mean age of the patients was 58.5 years (range, 22–83 years) at treatment (Table 1). There were seven men and three women. At presentation, seven patients complained of an ocular surface mass, and three patients complained of redness in the eye. The mean duration of ocular surface mass was 5.4 (range, 0.4–15) years. Five eyes had had previous resection of the OSN. The mean number of previous tumour resections was 1.8 (range, 1–5). Preoperative visual acuity ranged from hand motions to 20/20. Major reasons for decreased visual acuity before surgery were corneal involvement by the neoplasia and/or cataract.
Table 2 demonstrates preoperative tumour features, treatment details, histopathologic diagnosis, follow-up, limbal vascularization, fibrosis/symblepharon formation, and recurrence status. The mean largest tumour base diameter was 17.9 mm (range, 12–25 mm) and the mean tumour thickness was 2.4 mm (range, 1–5 mm). The tumour involved the bulbar conjunctiva for a mean of 7.0 (range, 5–12) clock hours. In nine of 10 cases, the tumour infiltrated the limbal conjunctiva for a mean of 7.0 (range, 4–12) clock hours. Extensive corneal involvement was present in four cases.
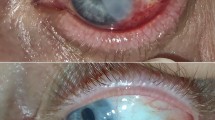
At presentation, the tumour involved the limbus, bulbar and fornicial conjunctiva in five eyes; the cornea, limbus, bulbar, and fornicial conjunctiva in four eyes (Figures 1a and 2a); and bulbar, fornicial, and tarsal conjunctiva in one eye (Figure 3a). In four eyes with corneal involvement, keratectomy was performed after excision of the OSN. Contact lenses were fitted in four eyes for periods of 15–30 days until corneal reepithelization was complete (Figure 1b and Table 2).
(a) Case 1: anterior segment photograph showing the conjunctival-corneal carcinoma in situ measuring 20 × 20 × 2 mm3 involving almost 360 degrees of limbus extending onto the cornea. (b) Case 1: anterior segment photograph the day after surgery demonstrating the amniotic membrane sutured to the cornea and the hydrogel lens fitted in place. The surgical intervention consisted of tumour excision, keratectomy, cryotherapy, and AMT. (c) Case 1: anterior segment photograph at postoperative month-27 showing the amniotic membrane successfully fitting in place with no symblepharon formation.
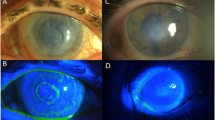
(a) Case 3: anterior segment photograph demonstrating 25 × 20 × 2 mm3 conjunctival–corneal carcinoma in situ covering almost the entire ocular surface including the cornea and extending to the inferior fornix. (b) Case 3: anterior segment photograph showing the successful anatomic outcome at postoperative month-6 following tumour excision, keratectomy, cryotherapy, and AMT. A superficial corneal vessel extends from the limbus towards the paracentral cornea inferonasally.
(a) Case 4: anterior segment photograph demonstrating 20 × 10 × 5 mm3 conjunuctival melanoma involving the limbus, bulbar conjunctiva, fornix, and tarsal conjunctiva. (b) Case 4: anterior segment photograph depicting the successful anatomic outcome at postoperative week-2 following tumour excision, cryotherapy, and AMT.
Histopathologic examination revealed that four patients had conjunctival-corneal carcinoma in situ, three patients had conjunctival squamous cell carcinoma, two had conjunctival malignant melanoma, and one had lymphangioma (Table 2). The patient with lymphangioma had previously undergone orbitotomy for orbital lymphangioma. Ocular surface reconstruction with AMT was performed to remove the cosmetically unsightly conjunctival lymphangioma.
The mean follow-up after surgical intervention was 10.0 months (range, 6–27 months (Table 2)). Postoperatively, visual acuity ranged from 20/100 to 20/20. Visual acuity increased in five (50%) eyes and remained unchanged in two (20%) eyes. Three eyes had 20/20 vision pre- and postoperatively. The reasons for nonincreasing visual acuity were cataract or macular scarring (Table 1).
Reconstruction of the ocular surface was achieved satisfactorily in all 10 eyes (Figures 1c, 2b and 3b). Two eyes (cases 2 and 3) with extensive and long-standing conjunctival carcinoma in situ had partial limbal stem cell deficiency characterized by superficial corneal vascularization and corneal opacification and one eye (case 3) developed mild symblepharon after tumour excision and AMT.
In the first few days following AMT, patients reported mild discomfort; when questioned at the last follow-up examination, patients had no complaints. Postoperatively, slit-lamp biomicroscopy and fluorescein staining revealed complete conjunctival epithelial healing with a smooth, stable, and wettable surface in all patients. No inflammation, infection, or immunologic rejection reaction to the nonpreserved amniotic membrane was observed.
In one eye with the histopathological diagnosis of conjunctival malignant melanoma, a 2 × 2 × 1 mm3 nonpigmented lesion thought to be a recurrence was noticed at the border of previous tumour excision at postoperative month 10. This small recurrence was totally resected in combination with double freeze-and-thaw cryotherapy. The histopathological examination of the recurrent lesion also revealed malignant melanoma. No further recurrence was observed during a further follow-up of 12 months. No tumour recurrence was observed in the other nine eyes.
None of the donors or recipient patients receiving human amniotic membrane were positive for hepatitis B, hepatitis C, or HIV the day before surgery or at 6 months follow-up.
Discussion
Amniotic membrane transplantation has been used in various medical specialties since 1910.9, 10, 11 Amniotic membrane has been used as a biological bandage for dressing burns and nonhealing skin ulcers; in the surgical reconstruction of the vagina, urethra, nasal mucosa, and tympanum; in repair of abdominal herniation, and in closure of pericardium.9, 10
In ophthalmology, the use of amnion was first reported by de Rötth in 1940 for treatment of conjunctival tissue loss.12 In 1946, Sorsby and Symons13 reported on the transplantation of amnion to treat chemical burns. In 1995, Kim and Tseng14 published on the use of AMT in ocular surface pathologies based on their work on animals. These authors utilized human amniotic membrane that was preserved in 100% glycerol and stored at 4°C.14 In 1997, Tseng and his colleagues reported on the use of amniotic membrane preserved in 50% Dulbecco's modified Eagle's medium and 50% glycerol and stored at −80°C in human subjects.15 This method of preservation and storage of amniotic membrane has since been used by many others. Recently, freeze-dried vacuum-packed amniotic membrane that can be stored at room temperature has become available.16
Amniotic membrane has three basic functions.10 First, it acts as a biological bandage protecting large areas of the underlying ocular surface in conditions like extensive chemical burns. The epithelium heals underneath the amniotic membrane in these circumstances. Second, it serves as a basement membrane transplant (substrate) allowing the epithelium to grow over it. The use of amniotic membrane in the reconstruction of the ocular surface for various pathologic conditions relates to its function as a basement membrane substrate. Third, it expresses growth factors and cytokines that promote epithelialization, suppress inflammation and fibrosis, and inhibit angiogenesis. Amniotic membrane increases epithelialization by producing a number of growth factors, including β-FGF (fibroblastic growth factor) and HGF (hepatocyte growth factor).17 It suppresses inflammation and fibrosis by downregulating the transforming growth factor-β (TGF-β) signalling system with the prevention of fibroblast activation into myofibroblasts.18, 19 Amniotic membrane also has antiangiogenic effects due to the expression of tissue inhibitors of metalloproteases and endostatin.20
In our study, we treated 10 eyes with extensive OSN using nonpreserved AMT. The use of nonpreserved AMT for various ocular surface diseases including OSN has been reported before.8, 21, 22, 23, 24 However, to our knowledge, the current study presents the largest series of patients with extensive OSN who had nonpreserved AMT for ocular surface reconstruction. There are potential, although not proven, advantages of using nonpreserved human amniotic membrane. If the effects of amnion are due to growth factors and cytokines, then nonpreserved amniotic membrane should, theoretically, be more effective than preserved membrane. Preserved amniotic membrane has no viable cells according to one study25 and approximately 50% viable cells according to another;26 therefore, the ability of preserved membrane to influence wound healing by growth factors must be nonexistent or limited since the epithelium is a major source for these growth factors. In fact, decreased amounts of growth factors were found in preserved human amniotic membrane without amniotic epithelium.27
The major concern with the use of nonpreserved amniotic membrane over preserved membrane is the risk of HIV infection despite seronegativity of the donor at the time of harvesting. Owing to the window period between infection and seroconversion, preserved amniotic membrane is released for use only if the donor is seronegative at the time of harvesting and at 6 months thereafter. None of our donors or patients were found seropositive for HIV, hepatitis B, or C the day before surgery or at 6 months postoperatively. Although rare, a risk exists that the donor could become infected shortly before harvesting, which could not be elicited in the serologic studies performed before surgery.
Another concern about using nonpreserved amniotic membrane is the risk of immune graft rejection. Human amniotic cells do not express HLA-A, B, C, or DR antigens on their surfaces.28 On the other hand, expression of HLA-G antigen (major histocompatibility complex class Ib) was found on amnion epithelium, mesenchymal cells, and fibroblasts.26 If viable epithelial cells are present after cryopreservation as reported previously,26 then there is at least a theoretical risk of immune graft rejection after transplantation of preserved amnion graft. On the other hand, human amniotic epithelial cells were reported to survive for 3 months after transplantation of nonpreserved amniotic membrane.24 In accordance with this data, we used topical steroids for 3 months postoperatively to control potential immune graft reaction. We observed no immune graft rejection in any eye with a nonpreserved amnion graft.
Infection of the recipient cornea after nonpreserved AMT has also been reported.29 The use of antibacterial and antifungal agents before amniotic membrane transplantation, as described previously,7 may decrease or eliminate the risk of infection.
Nonpreserved amniotic membrane transplantation was successful in the reconstruction of the ocular surface in all eyes in our series. We observed minimal fibrosis and symblepharon formation in one eye that had had extensive conjunctival carcinoma in situ. This symblepharon formation was not clinically significant and did not cause restriction of ocular movements, entropion, lagophthalmos, or ocular surface breakdown. Paridaens et al5 pointed out that in extensive ocular surface defects, the success of AMT might be limited. This is because the amniotic membrane acts as a substrate for migration, growth and differentiation of epithelial cells from the adjacent host conjunctiva.
Complete epithelialization of the amniotic membrane was achieved in all cases in our study. No surface or intraocular inflammation nor persistent epithelial defect or fluorescein staining was recorded in any eye during the follow-up period. Partial limbal stem cell deficiency was found in only two eyes despite the fact that limbal involvement was present in nine eyes and corneal involvement was present in four eyes. Previous studies showed that AMT yields successful results in restoring corneal surface in patients with partial limbal stem cell deficiency.14, 30 The amniotic membrane probably increases expansion of stem cells by creating a noninflamed perilimbal stromal environment due to its various effects, as discussed above.
In conclusion, nonpreserved human AMT appears to be useful for reconstruction of the ocular surface following excision of extensive OSN. Immune, infectious, or inflammatory reactions were not encountered in any eye in our study from the use of nonpreserved amniotic membrane. Further studies, with more patients and longer follow-up, are required to establish the role of nonpreserved AMT in the reconstruction of extensive conjunctival defects following surgical resection of OSN, as well as other pathologic conditions.
References
Shields JA, Shields CL, DePotter P . Surgical management of conjunctival tumors: the 1994 Lynn B. McMahan Lecture. Arch Ophthalmol 1997; 115: 808–815.
Vrabec MP, Weisenthal RW, Elsing SH . Subconjunctival fibrosis after conjunctival autograft. Cornea 1993; 12: 181–183.
Neuhaus RW, Baylis HI, Shorr N . Complications at mucous membrane donor sites. Am J Ophthalmol 1982; 93: 643–646.
Tseng SCG, Prabhasawat P, Lee SH . Amniotic membrane transplantation for conjunctival surface reconstruction. Am J Ophthalmol 1997; 124: 765–774.
Paridaens D, Beekhuis H, van den Bosch W, Remeyer L, Melles G . Amniotic membrane transplantation in the management of conjunctival malignant melanoma and primary acquired melanosis with atypia. Br J Ophthalmol 2001; 85: 658–661.
Espana EM, Prabhasawat P, Grueterich M, Solomon A, Tseng SCG . Amniotic membrane transplantation for reconstruction after excision of large ocular surface neoplasias. Br J Ophthalmol 2002; 86: 640–645.
Prabhasawat P, Tesavibul N . Preserved amniotic membrane transplantation for conjunctival surface reconstruction. CATB 2001; 2: 31–39.
Uçakhan ÖÖ, Köklü G, Fırat E . Nonpreserved human amniotic membrane transplantation in acute and chronic chemical eye injuries. Cornea 2002; 21: 169–172.
Dua HS, Azuaro-Blanco A . Amniotic membrane transplantation. Br J Ophthalmol 1999; 83: 748–752.
Dua HS, Gomes JAP, King AJ, Maharajan S . The amniotic membrane in ophthalmology. Surv Ophthalmol 2004; 49: 51–77.
Davis JW . Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J 1910; 15: 307. Cited by: Dua HS, Gomes JAP, King AJ, Maharajan S. The amniotic membrane in ophthalmology. Surv Ophthalmol 2004; 49: 51–77.
De Rötth A . Plastic repair of conjunctival defects with fetal membranes. Arch Ophthalmol 1940; 23: 522–525.
Sorsby A, Symons HM . Amniotic membrane grafts in caustic burns of the eye (burns of the second degree). Br J Ophthalmol 1946; 30: 337–345.
Kim JC, Tseng SCG . Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea 1995; 14: 473–484.
Lee SH, Tseng SCG . Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol 1997; 123: 303–312.
Nakamura T, Yoshitani M, Rigby H, Fullwood NJ, Ito W, Inatomi T et al. Sterilized, freeze-dried amniotic membrane: A useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci 2004; 45: 93–99.
Shimazaki J, Shinozaki N, Tsubota K . Transplantation of amniotic membrane and limbal autograft for patients with recurrent pterygium associated with symblepharon. Br J Ophthalmol 1998; 82: 235–240.
Tseng SCG, Li D-Q, Max X . Suppression of transforming growth factor isoforms, TGF-β receptor II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol 1999; 179: 325–335.
Lee S-B, Li D-Q, Tan DT, Meller DC, Tseng SC . Suppression of TGF-β signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Rec 2000; 20: 325–334.
Hao Y, Ma DH, Hwang DH, Kim WS, Zhang F . Identification of antiangiogenic and anti-inflammatory proteins in human amniotic membrane. Cornea 2000; 19: 348–352.
Mejia LF, Acosta C, Santamaria JP . Use of nonpreserved human amniotic membrane for the reconstruction of the ocular surface. Cornea 2000; 19: 288–291.
Panda A . Amniotic membrane transplantation in ophthalmology (fresh vs preserved tissue). Br J Ophthalmol 1999; 83: 1410–1411.
Batmanov IE, Egorova KS, Kolesnikova LN . The use of fresh amnion in the treatment of corneal diseases. Vestn Oftalmol 1990; 106: 17–19.
Zhou S, Chen J, Xu L, Lin J, Huang T . Fresh amniotic membrane transplantation for conjunctival surface reconstruction. Yan Ke Xue Bao 1999; 15: 169–173.
Kruse FE, Joussen AM, Rohrschneider K, You L, Sinn B, Baumann J et al. Cryopreserved human amniotic membrane for ocular surface reconstruction. Graefes Arch Clin Exp Ophthalmol 2000; 238: 68–75.
Kubo M, Sonada Y, Muramatsu R, Usui M . Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci 2001; 42: 1539–1546.
Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S . Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res 2000; 20: 173–177.
Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I . Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 1981; 7: 1003–1005.
Khokhar S, Sharma N, Kumar H, Soni A . Infection after use of nonpreserved human amniotic membrane for the reconstruction of the ocular surface. Cornea 2001; 20: 773–774.
Tseng SCG, Prabhasawat P, Barton K, Gray T, Meller D . Amniotic membrane transplantation with or without limbal autografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol 1998; 116: 431–441.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gündüz, K., Uçakhan, Ö., Kanpolat, A. et al. Nonpreserved human amniotic membrane transplantation for conjunctival reconstruction after excision of extensive ocular surface neoplasia. Eye 20, 351–357 (2006). https://doi.org/10.1038/sj.eye.6701890
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6701890
Keywords
This article is cited by
-
Ocular surface squamous neoplasia: outcomes following primary excision with 2 mm margin and cryotherapy
Eye (2021)
-
Fresh frozen amniotic membrane for conjunctival reconstruction after excision of neoplastic and presumed neoplastic conjunctival lesions
Eye (2017)
-
Long-term outcome of amniotic membrane transplantation combined with mitomycin C for conjunctival reconstruction after ocular surface squamous neoplasia excision
International Ophthalmology (2017)
-
Amniotic membrane transplantation in surgical management of ocular surface squamous neoplasias: long-term results
Eye (2014)
-
In vitro characterization and ex vivo surgical evaluation of human hair keratin films in ocular surface reconstruction after sterilization processing
Journal of Materials Science: Materials in Medicine (2013)