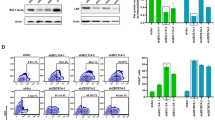
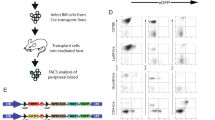
Abstract
Oncoretroviral vectors have been successfully used in gene therapy trials, yet low transduction rates and loss of transgene expression are still major obstacles for their application. To overcome these problems we modified the widely used Moloney murine leukemia virus-derived retroviral vector pMX by replacing the 3′LTR with the spleen focus-forming virus LTR and inserting the woodchuck hepatitis B virus post-translational regulatory element. To compare requirements crucial for efficient transgene expression, we generated the hybrid retroviral vectors pMOWS and pOWS that harbor the complete murine embryonic stem cell virus (MESV)-leader sequence or a shortened MESV-leader not comprising primer binding site (PBS) and splice donor (SD). Applying these retroviral vectors significantly augmented transgene expression in hematopoietic cell lines and progenitor cells. For transduction of murine embryonic stem (ES) cells the retroviral vector pMOWS that harbors the MESV-PBS and -SD was superior resulting in 65% green fluorescent protein (GFP) expressing ES cells. Surprisingly, in murine and human primitive hematopoietic progenitor cells (HPC), the highest efficiency of up to 66% GFP expressing cells was achieved with pOWS, a retroviral vector that retains the negative regulatory element coinciding with the MoMuLV-PBS. In summary our hybrid retroviral vectors facilitate significantly improved transgene expression in multipotent cells and thus possess great potential for reconstituting genes in primary cells of disease models, as well as for gene therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cavazzano-Calvo M. et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease Science 2000 288: 669 669
Daly G., Chernajovsky Y. . Recent developments in retroviral-mediated gene transduction Mol Ther 2000 2: 423 423
Onishi M. et al. Applications of retrovirus-mediated expression cloning Exp Hematol 1996 24: 324 324
Soudais C. et al. Stable and functional lymphoid reconstitution of common cytokine receptor γ chain deficient mice by retroviral-mediated gene transfer Blood 2000 95: 3071 3071
Baum C. et al. Novel retroviral vectors for efficient expression of the multidrug resistance (mdr-1) gene in early hematopoietic cells J Virol 1995 69: 7541 7541
Klug C.A., Cheshier S., Weissman I.L. . Inactivation of a GFP retrovirus occurs at multiple levels in long-term repopulating stem cells and their differentiated progeny Blood 2000 96: 894 894
Cherry S.R. et al. Retroviral expression in embryonic stem cells and hematopoietic stem cells Mol Cell Biol 2000 20: 7419 7419
Grez M., Akgün E., Hilberg F., Ostertag W. . Embryonic stem cell virus, a recombinant murine retrovirus with expression in embryonic stem cells Proc Natl Acad Sci USA 1990 87: 9202 9202
Kempler G. et al. Characterization of the Moloney murine leukemia virus stem cell- specific repressor binding site Virology 1993 193: 690 690
Loh T.P., Sievert L.L., Scott R.W. . Evidence for a stem cell-specific repressor of Moloney murine leukemia virus expression in embryonal carcinoma cells Mol Cell Biol 1990 10: 4045 4045
Baum C. et al. The potent enhancer activity of the polycythemic strain of spleen focus-forming virus in hematopoietic cells is governed by a binding site for Sp1 in the upstream control region and by a unique enhancer core motif, creating an exclusive target for PEBP/CBF J Virol 1997 71: 6323 6323
Hildinger M. et al. FMEV vectors: both retroviral long terminal repeat and leader are important for high expression in transduced hematopoietic cells Gene Therapy 1998 5: 1575 1575
Hildinger M., Abel K.L., Ostertag W., Baum C. . Design of 5’untranslated sequences in retroviral vectors developed for medical use J Virol 1999 73: 4083 4083
Zufferey R., Donello J.E., Trono D., Hope T.J. . Woodchuck hepatitis virus post-transcriptional regulatory element enhances expression of transgenes delivered by retroviral vectors J Virol 1999 73: 2886 2886
Schambach A. et al. Context dependence of different modules for post-transcriptional enhancement of gene expression from retroviral vectors Mol Ther 2000 2: 435 435
Krall W.J. et al. Increased levels of spliced RNA account for augmented expression from the MFG retroviral vector in hematopoietic cells Gene Therapy 1995 3: 37 37
Huang Y., Wimler K.M., Carmichael G.G. . Intronless mRNA transport elements may affect multiple steps of pre-mRNA processing EMBO J 1999 18: 1642 1642
Pear W.S., Nolan G.P., Scott M.L., Baltimore D. . Production of high-titer helper-free retroviruses by transient transfection Proc Natl Acad Sci USA 1993 90: 8392 8392
Robbins P.B. et al. Consistent, persistent expression from modified retroviral vectors in murine hematopoietic stem cells Proc Natl Acad Sci USA 1998 95: 10182 10182
Laker C. et al. Host cis-mediated extinction of a retrovirus permissive for expression in embryonal stem cells during differentiation J Virol 1998 72: 339 339
Robbins P.B. et al. Increased probability of expression from modified retroviral vectors in embryonal stem cells and embryonal carcinoma cells J Virol 1997 71: 9466 9466
Osborne C.S. et al. Amelioration of retroviral vector silencing in locus control region beta-globin-transgenic mice and transduced F9 embryonic cells J Virol 1999 73: 5490 5490
Lei H. et al. De novo DNA cytosine methyltransferase activities in mouse embryonic stem cells Development 1996 122: 3195 3195
Silke J. et al. Complex demethylation patterns at Sp1 binding sites in F9 embryonal carcinoma cells FEBS Lett 1995 370: 170 170
Glimm H. et al. Efficient serum-free retroviral gene transfer into primitive human hematopoietic progenitor cells by a defined high-titer, nonconcentrated vector-containing medium Hum Gene Ther 1998 9: 771 771
Dorrell C. et al. Expansion of human cord blood CD34+ CD38− cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function Blood 2000 95: 102 102
Briones J. et al. Retroviral gene transfer into human hematopoietic cells: an in vitro kinetic study Haematologica 1999 84: 483 483
Flasshove M. et al. Type and position of promoter elements in retroviral vectors have substantial effects on the expression level of an enhanced green fluorescent protein reporter gene J Cancer Res Clin Oncol 2000 126: 391 391
Fehse B. et al. CD34 splice variant: an attractive marker for selection of gene-modified cells Mol Ther 2000 1: 448 448
Limon A. et al. High-titer retroviral vectors containing the enhanced green fluorescent protein gene for efficient expression in hematopoietic cells Blood 1997 90: 3316 3316
Wahlers M. et al. Influence of multiplicity of infection and protein stability on retroviral vector-mediated gene expression in hematopoietic cells Gene Ther 2001 8: 477 477
Arai T., Takada M., Ui M., Iba H. . Dose-dependent transduction of vesicular stomatitis virus G protein-pseudotyped retrovirus vector into human solid tumor cell lines and murine fibroblasts Virology 1999 260: 109 109
Chida D., Miura O., Yoshimura A., Miyajima A. . Role of cytokine signaling molecules in eryhtroid differentiation of mouse fetal liver hematopoietic cells: functional analysis of signaling molecules by retrovirus-mediated expression Blood 1999 93: 1567 1567
Klingmüller U. et al. Identification of a novel pathway important for proliferation and differentiation of primary erythroid progenitors Proc Natl Acad Sci USA 1997 94: 3016 3016
Wu H. et al. Inactivation of erythropoietin leads to defects in cardiac morphogenesis Development 1999 126: 3597 3597
Wu H. et al. Functional interaction of erythropoietin and stem cell factor receptors is essential for erythroid colony formation Proc Natl Acad Sci USA 1997 94: 1806 1806
Acknowledgements
We thank Dr Christopher Baum (Heinrich-Pette-Institut für Experimentelle Virology und Immunologie, Universität Hamburg) for providing the retroviral vector pSFβ1, Dr Michael Nassal (Department of Internal Medicine II, University Hospital Freiburg) for supplying the woodchuck hepatitis virus vector pCWT, and Dr Albrecht Müller for providing antibodies against Ter119 and Gr1 and helpful advice with murine hematopoietic cell cultures. We are indebted to Dr Ira Swameye for helpful discussion, Melanie Wickert for technical assistance, and Dr Frank Reuss and Dr Randy Cassada for critically reading the manuscript. This work was supported by Sonderforschungsbereich 364 (Deutsche Forschungsgemeinschaft).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ketteler, R., Glaser, S., Sandra, O. et al. Enhanced transgene expression in primitive hematopoietic progenitor cells and embryonic stem cells efficiently transduced by optimized retroviral hybrid vectors. Gene Ther 9, 477–487 (2002). https://doi.org/10.1038/sj.gt.3301653
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301653
Keywords
This article is cited by
-
The iRhom homology domain is indispensable for ADAM17-mediated TNFα and EGF receptor ligand release
Cellular and Molecular Life Sciences (2021)
-
Cancer cell specific inhibition of Wnt/β-catenin signaling by forced intracellular acidification
Cell Discovery (2018)
-
Synthetic cytokine receptors transmit biological signals using artificial ligands
Nature Communications (2018)
-
IL-6/IL-12 Cytokine Receptor Shuffling of Extra- and Intracellular Domains Reveals Canonical STAT Activation via Synthetic IL-35 and IL-39 Signaling
Scientific Reports (2017)
-
Cleavage Site Localization Differentially Controls Interleukin-6 Receptor Proteolysis by ADAM10 and ADAM17
Scientific Reports (2016)