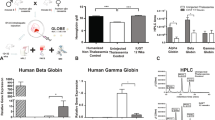
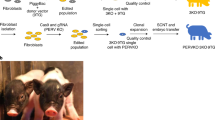
Abstract
Somatic gene delivery in utero is a novel approach to gene therapy for genetic disease based on the hypothesis that prenatal intervention may avoid the development of severe manifestations of early-onset disease, allow targeting of otherwise inaccessible tissues including expanding stem cell populations, induce tolerance against the therapeutic transgenic protein and thereby provide permanent somatic gene correction. This approach is particularly relevant in relation to prenatal screening programmes for severe genetic diseases as it could offer prevention as a third option to families faced with the prenatal diagnosis of a genetically affected child. Most investigations towards in utero gene therapy have been performed on mice and sheep fetuses as model animals for human disease and for the application of clinically relevant intervention techniques such as vector delivery by minimally invasive ultrasound guidance. Other animals such as dogs may serve as particular disease models and primates have to be considered in immediate preparation for clinical trials. Proof of principle for the hypothesis of fetal gene therapy has been provided during the last 2 years in mouse models for Crigler Najjar Disease, Leber's congenital amaurosis, Pompe's disease and haemophilia B showing long-term postnatal therapeutic effects and tolerance of the transgenic protein after in utero gene delivery. However, recently we have also observed a high incidence of liver tumours after in utero application of an early form of third-generation equine infectious anaemia virus vectors with SIN configuration. These findings highlight the need for more investigations into the safety and the ethical aspects of in utero gene therapy as well as for science-based public information on risks and benefits of this preventive gene therapy approach before application in humans can be contemplated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Coutelle C, Rodeck C . On the scientific and ethical issues of fetal somatic gene therapy. Gene Therapy 2002; 9: 670–673.
David A et al. The current status and future direction of fetal gene therapy. Gene Ther Mol Bio 2003; 7: 181–209.
Seppen J et al. Long-term correction of bilirubin UDPglucuronyltransferase deficiency in rats by in utero lentiviral gene transfer. Mol Ther 2003; 8: 593–599.
Dejneka NS et al. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol Ther 2004; 9: 182–188.
Rucker M et al. Rescue of enzyme deficiency in embryonic diaphragm in a mouse model of metabolic myopathy: Pompe disease. Development 2004; 131: 3007–3019.
Waddington S et al. Permanent phenotypic correction of haemophilia B in immunocompetent mice by prenatal gene therapy. Blood 2004; 104: 2714–2721.
Cremers F, van den Hurk JA, den Hollander AI . Molecular genetics of Leber congenital amaurosis. Hum Mol Genet 2002; 11: 1169–1176.
Waddington S et al. Long-term postnatal β-galactodsidase expression in transduced hepatocytes of immuno-competent mice after in utero delivery of an EAIV derived lentiviral vector via the yolk sac vessels. Gene Therapy 2003; 10: 1234–1240.
David A et al. Ultrasound guided percutaneous delivery of adenoviral vectors encoding the β-galactosidase and human factor IX genes to early gestation fetal sheep in utero. Hum Gene Ther 2003; 14: 353–364.
David A et al. Ultrasound-guided injection of the trachea in fetal sheep: a novel percutaneous technique to target the fetal airways in utero. Fetal Diag Ther 2003; 18: 385–390.
Peebles D et al. Widespread and efficient marker gene expression in the airway epithelia of fetal sheep after minimally invasive tracheal application of recombinant adenovirus in utero. Gene Therapy 2004; 11: 70–78.
Darrasse-Jeze GMG, Salomon BL, Catala M, Klatzmann D . Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetus. Blood 2005; 105: 4715–4721.
Carter AM, Enders AC . Comparative aspects of trophoblast development and placentation. Reprod Biol Endocrinol 2004; 2: 46.
Waddington S et al. In utero gene transfer of human factor IX to fetal mice can induce tolerance of the exogenous clotting factor. Blood 2003; 101: 1359–1366.
Lee CC et al. gene transfer using lentiviral vectors and the potential for germ cell transduction in rhesus monkeys (Macaca mulatta). Hum Gene Ther 2005; 16: 417–425.
Hacein-Bey-Abina S et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 2003; 348: 255–256.
Themis M et al. Oncogenesis following delivery of a non-primate lentiviral gene therapy vector to fetal mice. Mol Ther 2005; 12: 257–266.
Surbek DV et al. Ultrasound-guided stem cell sampling from the early ovine fetus for prenatal ex vivo gene therapy. Am J Obstet Gynecol 2002; 187: 960–963.
Waddington S et al. In utero gene therapy: current challenges and perspectives. Mol Ther 2005; 11: 661–676.
Gregory L et al. Highly efficient EIAV-mediated in utero gene transfer and expression in the major muscle groups affected by Duchenne muscular dystrophy. Gene Therapy 2004; 11: 1117–1125.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Coutelle, C., Themis, M., Waddington, S. et al. Gene Therapy Progress and Prospects: Fetal gene therapy – first proofs of concept – some adverse effects. Gene Ther 12, 1601–1607 (2005). https://doi.org/10.1038/sj.gt.3302632
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302632
Keywords
This article is cited by
-
An important step on the long path to clinical application of in utero gene therapy
Gene Therapy (2018)
-
Foetal surgery and using in utero therapies to reduce the degree of disability after birth. Could it be morally defensible or even morally required?
Medicine, Health Care and Philosophy (2017)
-
In utero stem cell transplantation and gene therapy: rationale, history, and recent advances toward clinical application
Molecular Therapy - Methods & Clinical Development (2016)
-
Systemic delivery of scAAV9 in fetal macaques facilitates neuronal transduction of the central and peripheral nervous systems
Gene Therapy (2013)
-
Efficient Gene Transfer Into the Mouse Lung by Fetal Intratracheal Injection of rAAV2/6.2
Molecular Therapy (2010)