Abstract
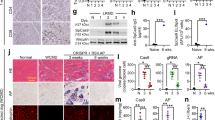
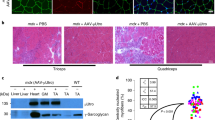
Using murine models, we have previously demonstrated that recombinant adeno-associated virus (rAAV)-mediated microdystrophin gene transfer is a promising approach to treatment of Duchenne muscular dystrophy (DMD). To examine further therapeutic effects and the safety issue of rAAV-mediated microdystrophin gene transfer using larger animal models, such as dystrophic dog models, we first investigated transduction efficiency of rAAV in wild-type canine muscle cells, and found that rAAV2 encoding β-galactosidase effectively transduces canine primary myotubes in vitro. Subsequent rAAV2 transfer into skeletal muscles of normal dogs, however, resulted in low and transient expression of β-galactosidase together with intense cellular infiltrations in vivo, where cellular and humoral immune responses were remarkably activated. In contrast, rAAV2 expressing no transgene elicited no cellular infiltrations. Co-administration of immunosuppressants, cyclosporine and mycophenolate mofetil could partially improve rAAV2 transduction. Collectively, these results suggest that immune responses against the transgene product caused cellular infiltration and eliminated transduced myofibers in dogs. Furthermore, in vitro interferon-γ release assay showed that canine splenocytes respond to immunogens or mitogens more susceptibly than murine ones. Our results emphasize the importance to scrutinize the immune responses to AAV vectors in larger animal models before applying rAAV-mediated gene therapy to DMD patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Emery AEH . Duchenne Muscular Dystrophy, 2nd edn. Oxford University Press: Oxford, 1993.
Fisher KJ, Jooss K, Alston J, Yang Y, Haecker SE, High K et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med 1997; 3: 306–312.
Yuasa K, Miyagoe Y, Yamamoto K, Nabeshima Y, Dickson G, Takeda S . Effective restoration of dystrophin-associated proteins in vivo by adenovirus-mediated transfer of truncated dystrophin cDNAs. FEBS Lett 1998; 425: 329–336.
Harper SQ, Hauser MA, Dellorusso C, Duan D, Crawford RW, Phelps SF et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med 2002; 8: 253–261.
Watchko J, O'day T, Wang B, Zhou L, Tang Y, Li J et al. Adeno-associated virus vector-mediated minidystrophin gene therapy improves dystrophic muscle contractile function in mdx mice. Hum Gene Ther 2002; 13: 1451–1460.
Fabb SA, Wells DJ, Serpente P, Dickson G . Adeno-associated virus vector gene transfer and sarcolemmal expression of a 144 kDa micro-dystrophin effectively restores the dystrophin-associated protein complex and inhibits myofibre degeneration in nude/mdx mice. Hum Mol Genet 2002; 11: 733–741.
Sakamoto M, Yuasa K, Yoshimura M, Yokota T, Ikemoto T, Suzuki M et al. Micro-dystrophin cDNA ameliorates dystrophic phenotypes when introduced into mdx mice as a transgene. Biochem Biophys Res Commun 2002; 293: 1265–1272.
Yuasa K, Sakamoto M, Miyagoe-Suzuki Y, Tanouchi A, Yamamoto H, Li J et al. Adeno-associated virus vector-mediated gene transfer into dystrophin-deficient skeletal muscles evokes enhanced immune response against the transgene product. Gene Therapy 2002; 9: 1576–1588.
Yoshimura M, Sakamoto M, Ikemoto M, Mochizuki Y, Yuasa K, Miyagoe-Suzuki Y et al. AAV vector-mediated microdystrophin expression in a relatively small percentage of mdx myofibers improved the mdx phenotype. Mol Ther 2004; 10: 821–828.
Shimatsu Y, Katagiri K, Furuta T, Nakura M, Tanioka Y, Yuasa K et al. Canine X-linked muscular dystrophy in Japan (CXMDJ). Exp Animals 2003; 52: 93–97.
Shimatsu Y, Yoshimura M, Yuasa K, Urasawa N, Tomohiro M, Nakura M et al. Major clinical and histopathological characteristies of canine X-linked muscular dystrophy in Japan, CXMDJ . Acta Myologica 2005; 24: 145–154.
Valentine BA, Cooper BJ, Cummings JF, De Lahunta A . Canine X-linked muscular dystrophy: morphologic lesions. J Neurol Sci 1990; 97: 1–23.
Manno CS, Arruda VR, Pierce GF, Glader B, Ragni M, Rasko J et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006; 12: 342–347.
Fisher KJ, Gao GP, Weitzman MD, DeMatteo R, Burda JF, Wilson JM . Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol 1996; 70: 520–532.
Herzog RW, Yang EY, Couto LB, Hagstrom JN, Elwell D, Fields PA et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med 1999; 5: 56–63.
Fields PA, Kowalczyk DW, Arruda VR, Armstrong E, Mccleland ML, Hagstrom JN et al. Role of vector in activation of T cell subsets in immune responses against the secreted transgene product factor IX. Mol Ther 2000; 1: 225–235.
Herzog RW, Mount JD, Arruda VR, High KA, Lothrop Jr CD . Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther 2001; 4: 192–200.
Herzog RW, Fields PA, Arruda VR, Brubaker JO, Armstrong E, Mcclintock D et al. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther 2002; 13: 1281–1291.
Arruda VR, Stedman HH, Nichols TC, Haskins ME, Nicholson M, Herzog RW et al. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood 2005; 105: 3458–3464.
Zhang Y, chirmule N, Gao G, Wilson J . CD40 ligand-dependent activation of cytotoxic T lymphocytes by adeno-associated virus vectors in vivo: role of immature dendritic cells. J. Virol 2000; 74: 8003–8010.
Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol 2005; 23: 321–328.
Inagaki K, Fuess S, Storm TA, Gibson GA, Mctiernan CF, Kay MA et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther 2006; 14: 45–53.
Xiao X, Li J, Samulski RJ . Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 1998; 72: 2224–2232.
Snyder R, Xiao X, Samulski RJ . Production of recombinant adeno-associated viral vectors. In: Dracopoli N, Haines J, Korf B, Morton C, Seidman C, Seidman JG, Smith D (eds). Current Protocols in Human Genetics. John Wiley & Sons Ltd: New York, 1996, pp 12.1.1–12.2.23.
Auricchio A, Hildinger M, O'connor E, Gao GP, Wilson JM . Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum Gene Ther 2001; 12: 71–76.
Howell JM, Fletcher S, Kakulas BA, O'hara M, Lochmuller H, Karpati G . Use of the dog model for Duchenne muscular dystrophy in gene therapy trials. Neuromuscul Disord 1997; 7: 325–328.
Howell JM, Lochmuller H, O'hara A, Fletcher S, Kakulas BA, Massie B et al. High-level dystrophin expression after adenovirus-mediated dystrophin minigene transfer to skeletal muscle of dystrophic dogs: prolongation of expression with immunosuppression. Hum Gene Ther 1998; 9: 629–634.
Rando TA, Blau HM . Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 1994; 125: 1275–1287.
Acknowledgements
We are grateful to Dr Katsujiro Sato, Dr Yasushi Mochizuki, Dr Naoko Yugeta, Dr Akiyo Nishiyama, Dr Madoka Ikemoto, Dr Michiko Wada, Ms Kazue Kinoshita, Ms Ryoko Nakagawa, Mr Satoru Masuda, Dr Masayuki Tomohiro and Dr Yoshiki Shimatsu for technical assistance. We also thank Mr Hideki Kita and Mr Shinichi Ichikawa and other staff of JAC Co. for care and management of experimental animals. This work is supported by Grants-in-Aid for Scientific Research for Research on Nervous and Mental Disorders (10B-1, 13B-1, 16B-3) and Health Sciences Research Grants for Research on Human Genome and Gene Therapy (H10-genome-015, H13-genome-001, H16-genome-003) from the Ministry of Heath, Labor and Welfare of Japan, and Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT). A part of this work in Musashino University is supported by High-Tech Research Center Project for Private Universities: matching fund subsidy from MEXT, 2004.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
Supplementary information
Rights and permissions
About this article
Cite this article
Yuasa, K., Yoshimura, M., Urasawa, N. et al. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther 14, 1249–1260 (2007). https://doi.org/10.1038/sj.gt.3302984
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302984
Keywords
This article is cited by
-
Serotype-specific transduction of canine joint tissue explants and cultured monolayers by self-complementary adeno-associated viral vectors
Gene Therapy (2023)
-
Gene therapy and gene correction: targets, progress, and challenges for treating human diseases
Gene Therapy (2022)
-
The golden retriever model of Duchenne muscular dystrophy
Skeletal Muscle (2017)
-
Oral-tolerization Prevents Immune Responses and Improves Transgene Persistence Following Gene Transfer Mediated by Adeno-associated Viral Vector
Molecular Therapy (2016)
-
Intra-Amniotic rAAV-Mediated Microdystrophin Gene Transfer Improves Canine X-Linked Muscular Dystrophy and May Induce Immune Tolerance
Molecular Therapy (2015)