Abstract
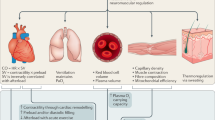
While medical therapies aim at reversing, reducing or eliminating diseases, the goal of enhancements is to improve performance or appearance beyond normal levels. Distinction between the two interventions is not always clear as they often present as a continuum. Gene therapy typically aims at treating or preventing disease, but the technology can theoretically be employed for enhancement. Some of the gene therapy enhancement strategies include improving performance by increasing muscle mass, endurance, memory, and cognition and bettering appearance by controlling weight, height and hair growth. In addition to the technical challenges of making enhancement strategies safe and effective, genetic enhancement presents significant ethical/societal questions that must be addressed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Miller H . Cat and mouse in regulating genetic ‘enhancement’. Nat Biotechnol 2005; 23: 171–172.
Wenz P . Engineering genetic injustice. Bioethics 2005; 19: 1–11.
The American Society for Aesthetic Plastic Surgery. 11.5 Million Cosmetic Procedures in 2006. (http://www.surgery.org/press/news-release.php?iid=465) last accessed 1/3/08.
Fuchs M . Gene therapy. An ethical profile of a new medical territory. J Gene Med 2006; 8: 1358–1362.
Haisma HJ, de HO . Gene doping. Int J Sports Med 2006; 27: 257–266.
Allen DB . Growth hormone therapy for short stature: is the benefit worth the burden? Pediatrics 2006; 118: 343–348.
Kimmelman J . Recent developments in gene transfer: risk and ethics. BMJ 2005; 330: 79–82.
Chan S, Harris J . The ethics of gene therapy. Curr Opin Mol Ther 2006; 8: 377–383.
O'Connor TP, Crystal RG . Genetic medicines: treatment strategies for hereditary disorders. Nat Rev Genet 2006; 7: 261–276.
Lebherz C, Auricchio A, Maguire AM, Rivera VM, Tang W, Grant RL et al. Long-term inducible gene expression in the eye via adeno-associated virus gene transfer in nonhuman primates. Hum Gene Ther 2005; 16: 178–186.
Walker MC, Mandell TC, Crawford PC, Simon GG, Cahill KS, Fernandes PJ et al. Expression of erythropoietin in cats treated with a recombinant adeno-associated viral vector. Am J Vet Res 2005; 66: 450–456.
Rivera VM, Gao GP, Grant RL, Schnell MA, Zoltick PW, Rozamus LW et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood 2005; 105: 1424–1430.
Voutetakis A, Bossis I, Kok MR, Zhang W, Wang J, Cotrim AP et al. Salivary glands as a potential gene transfer target for gene therapeutics of some monogenetic endocrine disorders. J Endocrinol 2005; 185: 363–372.
Stieger K, Le MG, Lasne F, Weber M, Deschamps JY, Nivard D et al. Long-term doxycycline-regulated transgene expression in the retina of nonhuman primates following subretinal injection of recombinant AAV vectors. Mol Ther 2006; 13: 967–975.
Mizukami H, Mimuro J, Ogura T, Okada T, Urabe M, Kume A et al. Adipose tissue as a novel target for in vivo gene transfer by adeno-associated viral vectors. Hum Gene Ther 2006; 17: 921–928.
Wang J, Voutetakis A, Papa M, Rivera VM, Clackson T, Lodde BM et al. Rapamycin control of transgene expression from a single AAV vector in mouse salivary glands. Gene Therapy 2006; 13: 187–190.
Oh TK, Quan GH, Kim HY, Park F, Kim ST . Correction of anemia in uremic rats by intramuscular injection of lentivirus carrying an erythropoietin gene. Am J Nephrol 2006; 26: 326–334.
Brzezinski M, Yanay O, Waldron L, Barry SC, Osborne WR . G-CSF-lentivirus administration in rats provided sustained elevated neutrophil counts and subsequent EPO-lentivirus administration increased hematocrits. J Gene Med 2007; 9: 571–578.
Fattori E, Cappelletti M, Zampaglione I, Mennuni C, Calvaruso F, Arcuri M et al. Gene electro-transfer of an improved erythropoietin plasmid in mice and non-human primates. J Gene Med 2005; 7: 228–236.
Richard P, Pollard H, Lanctin C, Bello-Roufai M, Desigaux L, Escande D et al. Inducible production of erythropoietin using intramuscular injection of block copolymer/DNA formulation. J Gene Med 2005; 7: 80–86.
Sebestyen MG, Hegge JO, Noble MA, Lewis DL, Herweijer H, Wolff JA . Progress toward a nonviral gene therapy protocol for the treatment of anemia. Hum Gene Ther 2007; 18: 269–285.
Brill-Almon E, Stern B, Afik D, Kaye J, Langer N, Bellomo S et al. Ex vivo transduction of human dermal tissue structures for autologous implantation production and delivery of therapeutic proteins. Mol Ther 2005; 12: 274–282.
Lippin Y, Dranitzki-Elhalel M, Brill-Almon E, Mei-Zahav C, Mizrachi S, Liberman Y et al. Human erythropoietin gene therapy for patients with chronic renal failure. Blood 2005; 106: 2280–2286.
Orive G, De CM, Ponce S, Hernandez RM, Gascon AR, Bosch M et al. Long-term expression of erythropoietin from myoblasts immobilized in biocompatible and neovascularized microcapsules. Mol Ther 2005; 12: 283–289.
Eliopoulos N, Gagnon RF, Francois M, Galipeau J . Erythropoietin delivery by genetically engineered bone marrow stromal cells for correction of anemia in mice with chronic renal failure. J Am Soc Nephrol 2006; 17: 1576–1584.
Schakman O, Gilson H, de Coninck V, Lause P, Verniers J, Havaux X et al. Insulin-like growth factor-I gene transfer by electroporation prevents skeletal muscle atrophy in glucocorticoid-treated rats. Endocrinology 2005; 146: 1789–1797.
Abmayr S, Gregorevic P, Allen JM, Chamberlain JS . Phenotypic improvement of dystrophic muscles by rAAV/microdystrophin vectors is augmented by Igf1 codelivery. Mol Ther 2005; 12: 441–450.
Kusano K, Tsutsumi Y, Dean J, Gavin M, Ma H, Silver M et al. Long-term stable expression of human growth hormone by rAAV promotes myocardial protection post-myocardial infarction. J Mol Cell Cardiol 2007; 42: 390–399.
Acosta J, Carpio Y, Borroto I, Gonzalez O, Estrada MP . Myostatin gene silenced by RNAi show a zebrafish giant phenotype. J Biotechnol 2005; 119: 324–331.
Bartoli M, Poupiot J, Vulin A, Fougerousse F, Arandel L, Daniele N et al. AAV-mediated delivery of a mutated myostatin propeptide ameliorates calpain 3 but not alpha-sarcoglycan deficiency. Gene Therapy 2007; 14: 733–740.
Nicholas A, Munhoz CD, Ferguson D, Campbell L, Sapolsky R . Enhancing cognition after stress with gene therapy. J Neurosci 2006; 26: 11637–11643.
Zhang GR, Wang X, Kong L, Lu XG, Lee B, Liu M et al. Genetic enhancement of visual learning by activation of protein kinase C pathways in small groups of rat cortical neurons. J Neurosci 2005; 25: 8468–8481.
Boghossian S, Lecklin A, Torto R, Kalra PS, Kalra SP . Suppression of fat deposition for the life time with gene therapy. Peptides 2005; 26: 1512–1519.
Scarpace PJ, Matheny M, Tumer N, Cheng KY, Zhang Y . Leptin resistance exacerbates diet-induced obesity and is associated with diminished maximal leptin signalling capacity in rats. Diabetologia 2005; 48: 1075–1083.
Lecklin A, Dube MG, Torto RN, Kalra PS, Kalra SP . Perigestational suppression of weight gain with central leptin gene therapy results in lower weight F1 generation. Peptides 2005; 26: 1176–1187.
Otukonyong EE, Dube MG, Torto R, Kalra PS, Kalra SP . Central leptin differentially modulates ultradian secretory patterns of insulin, leptin and ghrelin independent of effects on food intake and body weight. Peptides 2005; 26: 2559–2566.
Ueno N, Inui A, Kalra PS, Kalra SP . Leptin transgene expression in the hypothalamus enforces euglycemia in diabetic, insulin-deficient nonobese Akita mice and leptin-deficient obese ob/ob mice. Peptides 2006; 27: 2332–2342.
Boghossian S, Dube MG, Torto R, Kalra PS, Kalra SP . Hypothalamic clamp on insulin release by leptin-transgene expression. Peptides 2006; 27: 3245–3254.
Boghossian S, Lecklin A, Dube MG, Kalra PS, Kalra SP . Increased leptin expression in the dorsal vagal complex suppresses adiposity without affecting energy intake and metabolic hormones. Obesity (Silver Spring) 2006; 14: 1003–1009.
Torto R, Boghossian S, Dube MG, Kalra PS, Kalra SP . Central leptin gene therapy blocks ovariectomy-induced adiposity. Obesity (Silver Spring) 2006; 14: 1312–1319.
Boghossian S, Ueno N, Dube MG, Kalra P, Kalra S . Leptin gene transfer in the hypothalamus enhances longevity in adult monogenic mutant mice in the absence of circulating leptin. Neurobiol Aging 2007; 28: 1594–1604.
Scarpace PJ, Matheny M, Zhang Y, Cheng KY, Tumer N . Leptin antagonist reveals an uncoupling between leptin receptor signal transducer and activator of transcription 3 signaling and metabolic responses with central leptin resistance. J Pharmacol Exp Ther 2007; 320: 706–712.
Li G, Zhang Y, Wilsey JT, Scarpace PJ . Hypothalamic pro-opiomelanocortin gene delivery ameliorates obesity and glucose intolerance in aged rats. Diabetologia 2005; 48: 2376–2385.
Li G, Zhang Y, Rodrigues E, Zheng D, Matheny M, Cheng KY et al. Melanocortin activation of nucleus of the solitary tract avoids anorectic tachyphylaxis and induces prolonged weight loss. Am J Physiol Endocrinol Metab 2007; 293: E252–E258.
Li G, Zhang Y, Cheng KY, Scarpace PJ . Lean rats with hypothalamic pro-opiomelanocortin overexpression exhibit greater diet-induced obesity and impaired central melanocortin responsiveness. Diabetologia 2007; 50: 1490–1499.
Tumer N, Scarpace PJ, Dogan MD, Broxson CS, Matheny M, Yurek DM et al. Hypothalamic rAAV-mediated GDNF gene delivery ameliorates age-related obesity. Neurobiol Aging 2006; 27: 459–470.
Lou H, Crystal RG, Leopold PL . Enhanced efficacy of cholesterol-minus sonic hedgehog in postnatal skin. Mol Ther 2005; 12: 575–578.
The World Anti-doping Code. The 2006 Prohibited List. (http://www.wada-ama.org/rtecontent/document/2006_LIST.pdf) last accessed 1/3/08.
Gene Doping. Play True—An Official Publication of the World Anti-Doping Agency, 2005. (http://www.wada-ama.org/rtecontent/document/Play_True_01_2005_en.pdf) last accessed 1/3/08.
Azzazy HM, Mansour MM, Christenson RH . Doping in the recombinant era: strategies and counterstrategies. Clin Biochem 2005; 38: 959–965.
Lippi G, Franchini M, Salvagno GL, Guidi GC . Biochemistry, physiology, and complications of blood doping: facts and speculation. Crit Rev Clin Lab Sci 2006; 43: 349–391.
Miah A . Rethinking enhancement in sport. Ann NY Acad Sci 2006; 1093: 301–320.
Trent RJ, Alexander IE . Gene therapy in sport. Br J Sports Med 2006; 40: 4–5.
Baoutina A, Alexander IE, Rasko JE, Emslie KR . Potential use of gene transfer in athletic performance enhancement. Mol Ther 2007; 15: 1751–1766.
Diamanti-Kandarakis E, Konstantinopoulos PA, Papailiou J, Kandarakis SA, Andreopoulos A, Sykiotis GP . Erythropoietin abuse and erythropoietin gene doping: detection strategies in the genomic era. Sports Med 2005; 35: 831–840.
Heinicke K, Baum O, Ogunshola OO, Vogel J, Stallmach T, Wolfer DP et al. Excessive erythrocytosis in adult mice overexpressing erythropoietin leads to hepatic, renal, neuronal, and muscular degeneration. Am J Physiol Regul Integr Comp Physiol 2006; 291: R947–R956.
Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J . Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol 2007; 49: 1015–1026.
Xie D, Li Y, Reed EA, Odronic SI, Kontos CD, Annex BH . An engineered vascular endothelial growth factor-activating transcription factor induces therapeutic angiogenesis in ApoE knockout mice with hindlimb ischemia. J Vasc Surg 2006; 44: 166–175.
Alton E, Ferrari S, Griesenbach U . Progress and prospects: gene therapy clinical trials (part 1). Gene Therapy 2007; 14: 1439–1447.
Bassel-Duby R, Olson EN . Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem 2006; 75: 19–37.
Schakman O, Thissen JP . Gene therapy with anabolic growth factors to prevent muscle atrophy. Curr Opin Clin Nutr Metab Care 2006; 9: 207–213.
Walenkamp MJ, Wit JM . Genetic disorders in the growth hormone—insulin-like growth factor-I axis. Horm Res 2006; 66: 221–230.
Matsakas A, Diel P . The growth factor myostatin, a key regulator in skeletal muscle growth and homeostasis. Int J Sports Med 2005; 26: 83–89.
Joulia-Ekaza D, Cabello G . Myostatin regulation of muscle development: molecular basis, natural mutations, physiopathological aspects. Exp Cell Res 2006; 312: 2401–2414.
Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci USA 2005; 102: 18117–18122.
Grimaldi PA . Regulatory role of peroxisome proliferator-activated receptor delta (PPAR delta) in muscle metabolism. A new target for metabolic syndrome treatment? Biochimie 2005; 87: 5–8.
Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y et al. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab 2007; 5: 35–46.
Hautala AJ, Leon AS, Skinner JS, Rao DC, Bouchard C, Rankinen T . Peroxisome proliferator-activated receptor-delta polymorphisms are associated with physical performance and plasma lipids: the HERITAGE Family Study. Am J Physiol Heart Circ Physiol 2007; 292: H2498–H2505.
Takeshima Y, Yagi M, Wada H, Ishibashi K, Nishiyama A, Kakumoto M et al. Intravenous infusion of an antisense oligonucleotide results in exon skipping in muscle dystrophin mRNA of Duchenne muscular dystrophy. Pediatr Res 2006; 59: 690–694.
Daniel JM . Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol 2006; 18: 787–795.
Kalra SP, Kalra PS . Gene-transfer technology: a preventive neurotherapy to curb obesity, ameliorate metabolic syndrome and extend life expectancy. Trends Pharmacol Sci 2005; 26: 488–495.
Lee JM, Howell JD . Tall girls: the social shaping of a medical therapy. Arch Pediatr Adolesc Med 2006; 160: 1035–1039.
Peroni CN, Gout PW, Bartolini P . Animal models for growth hormone gene therapy. Curr Gene Ther 2005; 5: 493–509.
Vogt A, Mandt N, Lademann J, Schaefer H, Blume-Peytavi U . Follicular targeting—a promising tool in selective dermatotherapy. J Investig Dermatol Symp Proc 2005; 10: 252–255.
Hoffman RM . The hair follicle and its stem cells as drug delivery targets. Expert Opin Drug Deliv 2006; 3: 437–443.
Sugiyama-Nakagiri Y, Akiyama M, Shimizu H . Hair follicle stem cell-targeted gene transfer and reconstitution system. Gene Therapy 2006; 13: 732–737.
Alton E, Ferrari S, Griesenbach U . Progress and prospects: gene therapy clinical trials (part 2). Gene Therapy 2007; 14: 1555–1563.
Mavilio F, Pellegrini G, Ferrari S, Di NF, Di IE, Recchia A et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med 2006; 12: 1397–1402.
Whitehouse PJ, Juengst ET . Antiaging medicine and mild cognitive impairment: practice and policy issues for geriatrics. J Am Geriatr Soc 2005; 53: 1417–1422.
Marshall J . Life extension research: an analysis of contemporary biological theories and ethical issues. Med Health Care Philos 2006; 9: 87–96.
Acknowledgements
We thank N Mohamed for her help in preparing this paper. These studies were supported, in part, by U01 HL66952; U01 NS047458; P01 HL51746 and the Will Rogers Memorial Fund, Los Angeles, CA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiuru, M., Crystal, R. Progress and prospects: gene therapy for performance and appearance enhancement. Gene Ther 15, 329–337 (2008). https://doi.org/10.1038/sj.gt.3303100
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3303100
Keywords
This article is cited by
-
Ethical Issues of Using CRISPR Technologies for Research on Military Enhancement
Journal of Bioethical Inquiry (2018)
-
Risk, Russian-roulette and lotteries: Persson and Savulescu on moral enhancement
Medicine, Health Care and Philosophy (2013)
-
Progress & Prospects: Gene therapy in aging
Gene Therapy (2009)