Abstract
Objective:
We compared two methods of delivering high-flow gas therapy by nasal cannula, applied immediately after planned endotracheal extubations of NICU patients.
Study design and methods:
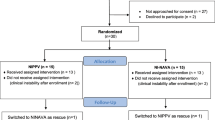
Thirty NICU patients who were about to be extubated from mechanical ventilation were randomized into two groups; Group 1 received Vapotherm® for the first 24 h after extubation, then standard high-flow nasal cannula for the next 24 h, and Group 2 received standard high-flow therapy for the first 24 h, then Vapotherm® for the next 24 h. At 24 h after extubation and again 48 h after extubation, a neonatologist who was not aware which modality the patient had been receiving examined the nasal mucosa and applied a scoring system. A research nurse who was unaware of the modality abstracted respiratory rates and respiratory effort scores from a specific study-bedside record. The experimental design was such that a patient could ‘fail’ extubation either by reintubation for mechanical ventilation, or by rescue to the opposite modality before completing the 24-h test period.
Results:
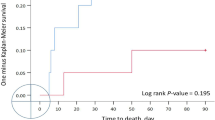
Fifteen patients were randomized to Group 1 and 15 to Group 2. No differences were apparent between the groups in birth weight, gestational age, age at study entry, gender or underlying pulmonary disorder. Respiratory rates were similar while on Vapotherm® (52±13 breaths/min, mean±s.d.) and high-flow (54±14/min). At 24 h after starting the modality, those on Vapotherm® had more normal examinations of the nasal mucosa (2.7±1.2 vs 7.8±1.7, P<0.0005) and lower respiratory effort scores (1.2±0.6 vs 2.0±0.9, P<0.05) than did those on high-flow. No patients failed while on Vapotherm®, but seven failed while on high-flow (two reintubations and five rescue switches to Vapotherm®, P<0.005).
Conclusions:
Among NICU patients immediately following extubation, Vapotherm® performed better than a standard high-flow nasal cannula in maintaining a normal appearing nasal mucosa, a lower respiratory effort score, and averting reintubation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sreenan C, Lemke RP, Hudson-Mason A, Osiovich H . High-flow nasal cannulae in the management of apnea of prematurity: a comparison with conventional nasal continuous positive airway pressure. Pediatrics 2001; 107: 1081–1083.
Myers TR . AARC clinical practice guideline. Selection of an oxygen delivery devise for neonatal and pediatric patients. Respir Care 2002; 47: 707–716.
Kopelman A, Holbert D . Use of oxygen cannulas in extremely low birth weight infants is associated with mucosal trauma and bleeding, and possibly with coagulase-negative staphylococcal sepsis. J Perinatol 2003; 23: 69–97.
In-house literature. Vapotherm 2004. Inc. 198 Log Canoe Circle: Stevensville, MD.
Waugh JB, Granger WM . An evaluation of 2 new devices for nasal high-flow gas therapy. Respir Care 2004; 49: 902–906.
A Collective Task Force Facilitated by the American College of Chest Physicians, the American Association for Respiratory Care, and the American College of Critical Care Medicine. Evidence-based guidelines for weaning and discontinuing ventilatory support. Respir Care 2002; 47: 69–90.
Durbin Jr CG, Campbell RS, Bransone RD . AARC clinical practice guideline. Removal of the endotracheal tube. Respir Care 1999; 44: 85–90.
deKlerk A . Humidified high flow nasal cannulae. Hot Topics Neonatol 2005; 4–6: 138–158.
Centers for Disease Control and Prevention. Ralstonia associated with Vapotherm oxygen delivery device – United States, 2005. Morb Mortal Wkly Rep 2005; 54: 1052–1053.
Locke RG, Wolfson MR, Shaffer TH, Rubinstein SD, Greenspan JS . Inadvertent administration of positive-end-distending pressure during nasal cannula flow. Pediatrics 1993; 91: 135–138.
Finer NN . Nasal cannula use in the preterm infant: oxygen or pressure? Pediatrics 2005; 116: 1216–1217.
Acknowledgements
The authors thank the nurses and respiratory therapists at the McKay-Dee Hospital NICU for their valuable assistance with the study. We also thank Gorgi Rigby, RRT, LDS Hospital NICU, Salt Lake City, UT for helpful discussions about the research project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woodhead, D., Lambert, D., Clark, J. et al. Comparing two methods of delivering high-flow gas therapy by nasal cannula following endotracheal extubation: a prospective, randomized, masked, crossover trial. J Perinatol 26, 481–485 (2006). https://doi.org/10.1038/sj.jp.7211543
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7211543
Keywords
This article is cited by
-
Adjustment of high flow nasal cannula rates using real-time work of breathing indices in premature infants with respiratory insufficiency
Journal of Perinatology (2021)
-
Differential impact of flow and mouth leak on oropharyngeal humidification during high-flow nasal cannula: a neonatal bench study
World Journal of Pediatrics (2018)
-
Nasal high flow treatment in preterm infants
Maternal Health, Neonatology and Perinatology (2017)
-
Consensus approach to nasal high-flow therapy in neonates
Journal of Perinatology (2017)
-
The effects of a 2-h trial of high-flow oxygen by nasal cannula versus Venturi mask in immunocompromised patients with hypoxemic acute respiratory failure: a multicenter randomized trial
Critical Care (2015)