Abstract
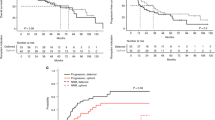
Since graft-versus-leukemia (GVL) is the main weapon for disease eradication after reduced intensity conditioning (RIC) allogeneic SCT, the availability of sensitive and specific techniques to monitor changes in tumor load after transplant are especially helpful. These minimal residual disease techniques would allow an early intervention in the event of low tumor burden, for which immunotherapy is highly effective. Some authors have found an association between persistence of MRD, mixed chimerism and risk of relapse. Nevertheless, data from the literature remain contradictory and further correlations should be established, especially in RIC transplants. In this study we have analyzed the impact of MRD and chimerism monitoring on the outcome of 34 patients undergoing RIC allogeneic SCT who were considered poor candidates for conventional transplantation due to advanced age or other concurrent medical conditions. At day +100 25 (75%) patients reached complete remission (CR), there were five (15%) partial responses and three patients progressed. Incidence of grade 2–4 aGVHD and extensive cGVHD were 35% and 58%, respectively. Sixteen percent of patients developing aGVHD relapsed as compared to 47% in those without aGVHD (P = 0.03) and also 10% of patients developing cGVHD relapsed as compared to 50% relapses in those without cGHVD (P = 0.03). Four patients (12%) died due to early (n = 1) and late (n = 3) transplant-related mortality. After a median follow-up of 15 months, 24 out of the 34 patients remain alive. Projected overall survival and disease-free survival at 3 years are 68% and 63%, respectively. Early chimerism analysis showed 67% of patients with complete chimerism (CC) in bone marrow (BM), 86% in peripheral blood (PB), 89% in granulocytes and 68% in T lymphocytes. On day +100, these figures were 68%, 79%, 90% and 73%, respectively, and on day +180 there were 83% patients with CC in BM, 100% in PB, 100% in granulocytes and 100% in T lymphocytes. We observed a trend to a higher incidence of relapse in patients with mixed chimerism (MC) as compared to patients with CC. MRD monitoring by flow cytometry and/or RT-PCR analysis was performed in 23 patients. MRD assessment on days +21 to +56 after transplant allowed identification of patients at risk of relapse. In this sense, seven out of 12 patients (58.3%) who had positive MRD on days +21 to +56 relapsed as compared to none out of 11 patients who had negative MRD (P = 0.002). Of the seven patients with criteria to monitor MRD who relapsed after transplant, all but one remained MRD positive until relapse. By contrast, 10 patients remained MRD negative and all of them are in continuous CR. In nine additional patients, persistence of MRD or mixed chimerism was observed after transplant and withdrawal of cyclosporin with or without DLI was performed. Only two out of these nine patients relapsed. MRD clearance was preceded by CC and GVHD. In conclusion, in our study we found that RIC allogeneic transplantation can be used in patients considered poor candidates for conventional transplantation due to advanced age or other concurrent medical conditions with both low toxicity and low transplant-related mortality. Simultaneous studies of both chimerism and MRD are a useful tool in order to predict risk of relapse in patients undergoing RIC transplants and so can be helpful for individualizing treatment strategies after transplant.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Advisory Committee of the International Bone Marrow Transplant Registry. Report from the International Bone Marrow Transplant Registry Bone Marrow Transplant 1989 48: 453–458
Bortin MM, Horowitz MM, Gale RP, Barret AJ, Champlin RE, Dicke KA, Gluckman E, Kolb HJ, Marmont AM, Mrsic M . Changing trends in allogeneic bone marrow transplantation for leukemia in the 1980s JAMA 1992 268: 607–612
Armitage JO . Bone marrow transplantation N Engl J Med 1994 330: 827–838
Mackinnon S . Who may benefit from donor leucocyte infusions after allogeneic stem cell transplantation? Br J Haematol 2000 110: 12–17
Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, Varadi G, Kirschbaum M, Ackerstein A, Samuel S, Amar A, Brautbar C, Ben-Tal O, Eldor A, Or R . Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases Blood 1998 91: 756–763
Giralt S, Estey E, Albitar M, van Besien K, Rondon G, Anderlini P, O'Brien S, Khouri I, Gajewski J, Mehra R, Claxton D, Andersson B, Beran M, Przepiorka D, Koller C, Kornblau S, Körbling M, Keating M, Kantarjian H, Champlin R . Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft versus leukemia without myeloablative therapy Blood 1997 89: 4531–4536
Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem H-P, Leisenring W, Shulman H . Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation Blood 1997 89: 3048–3054
San Miguel JF, van Dongen JJM . Methods for detection of minimal residual disease In: Buchner T et al (eds) Acute Leukemias VI. Springer Verlag: Berlin 1997 pp 307–312
Porter DL, Roth MS, McGarigle C, Ferrara JL, Antin JH . Induction of graft versus host disease as immunotherapy for relapsed chronic myeloid leukemia N Engl J Med 1994 330: 100–106
Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringden O, Rozman C, Speck B . Graft versus leukemia reactions after bone marrow transplantation Blood 1990 75: 555–562
Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED . Antileukemic effect of chronic graft versus host disease: contribution to improved survival after allogeneic marrow transplantation N Engl J Med 1981 304: 1529–1533
Collins RH Jr, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, Goodman SA, Wolff SN, Hu W, Verfaillie C, List A, Dalton W, Ognoskie N, Chetrit A, Antin JH, Nemunaitis J . Donor leukocyte infusions in 140 patients relapsed with malignancy after allogeneic bone marrow transplantation J Clin Oncol 1997 15: 433–444
Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A, Verdonck L, Niederwieser D . Graft versus leukemia effect of donor lymphocyte transfusions in marrow grafted patients Blood 1995 86: 2041–2050
Bertz H, Burger JA, Kunzmann R, Mertelsmann R, Finke J . Adoptive immunotherapy for relapsed multiple myeloma after allogeneic bone marrow transplantation (BMT): evidence for a graft versus myeloma effect Leukemia 1997 11: 281–283
Mackinnon S, Papadopoulos EP, Carabasi MH, Reich L, Collins NH, Poulad F, Castro-Malaspina H, Childs BH, Gilio AP, Kernan NA, Small TN, Young JW, O'Reilly RJ . Adoptive immunotherapy evaluating escalating doses of donor leucocytes for relapse of chronic myeloic leukemia following bone marrow transplantation: separation of graft versus leukemia responses from graft versus host disease Blood 1995 86: 1261–1268
Mackinnon S, Barnett L, Heller G, O'Reilly RJ . Minimal residual disease is more common in patients who have mixed T-cell chimerism after bone marrow transplantation for chronic myelogenous leukemia Blood 1994 83: 3409–3416
Roux E, Helg C, Chapuis B . Mixed chimerism after bone marrow transplantation and the risk of relapse Blood 1994 83: 4385–4386
van Leeuwen JEM, van Tol MJD, Joosten AM, Wijnen JT, Khan PM, Vossen JM . Mixed T-lymphoid chimerim after allogeneic bone marrow transplantation for hematologic malignancies of children is not correlated with relapse Blood 1993 82: 1921–1928
Mackinnon S, Barnett L, Bourhis JH, Black P, Heller G, O'Reilly RJ . Myeloid and lymphoid chimaerism after T-cell depleted bone marrow transplantation: evaluation of conditioning regimens using the polymerase chain reaction to amplify human minisatellite regions of genomic DNA Blood 1992 80: 3235–3241
van Leeuwen JEM, van Tol MJD, Joosten AM, Wijnen JT, Verweij PJ, Khan PM, Vossen JM . Persistence of host type hematopoiesis after allogeneic bone marrow transplantation for leukemia is significantly related to the recipient's age and/or the conditioning regimen, but is not associated with an increased risk of relapse Blood 1994 83: 3059–3067
Quantitative analysis of chimerism after allogeneic stem cell transplant using multiple PCR amplification of short tandem repeat markers and fluorescence detection. Appendix: method in focus Leukemia 2001 15: 303–306
van Dongen JJM, Macintyre EA, Gabert JA, Delabesse E, Rossi V, Saglio G, Gottardi E, Rambaldi A, Dotti G, Griesinger F, Parreira A, Gameiro P, Díaz MG, Malec M, Langerak AW, San Miguel JF, Biondi A . Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease Leukemia 1999 13: 1901–1928
Chillón MC, García-Sanz R, Balanzategui A, Ramos F, Fernández-Calvo J, Rodriguez MJ, Rodriguez-Salazar MI, Corrales A, Calmuntia MJ, Orfao A, González M, San Miguel JF . Molecular characterization of acute myeloblastic leukemia according to the new WHO classification : a different distribution in Central-West Spain Haematologica 2001 86: 162–166
San Miguel JF, Martínez A, Macedo A, Vidriales MB, López-Berges C, Gonzalez M, Caballero D, Gacía-Marcos MA, Ramos F, Pernández-Calvo J, Calmuntia MJ, Diaz-Mediavill J, Orfao A . Immunophenotyping investigation of minimal residual disease is a useful approach for predicting relapse in acute myeloid leukemia patients Blood 1997 90: 2465–2470
San Miguel JF, Almeida J, Ocqueteau M, Mateo G, Caballero MD, García-Sanz R, González M, Corral M, Bladé J, Hernández JM, Orfao A . Immunophenotyping and DNA ploidy analysis for detection of Minimal Residual Disease in MM Cancer Res Ther Control 1998 6: 299–302
Ciudad J, San Miguel JF, López-Berges MC, Vidrials B, Valverde B, Ocqueteau M, Caballero MD, Hernandez J, Moro MJ, Mateos MV, Orfao A . Prognostic value of immunophenotypic detection of minimal residula disease in acute lymphoblastic leukemia J Clin Oncol 1998 16: 3774–3781
San Miguel JF, Ciudad J, Vidriales MB, Orfao A, Lucio P, Porwitt MacDonald A . Immunophenotypical detection of minimal residual disease in acute leukemia Crit Rev Oncol Hematol 1999 32: 175–185
Clift R, Appelbaum F, Thomas E . Treatment of chronic myeloid leukemia by marrow transplantation Blood 1993 82: 1954–1956
Klingemann HG, Storb R, Fefer A, Deeg HJ, Appelbaum F, Buckner CD, Cheever MA, Greenberg PD, Stewart PS, Sullivan KM, Whitherspoon RP, Thomas ED . Bone marrow transplantation in patients aged 45 years and older Blood 1986 65: 770–776
Ringden O, Horwitz MH, Gale RP, Biggs JC, Gajewski J, Rimm AA, Speck B, Veum-Stone JA, de Witte T, Bortin MM . Outcome after allogeneic bone marrow transplantation for leukemia in older patients JAMA 193 270: 57–60
Blumme KG, Forman SJ, Nademanee AP, O'Donnell MR, Snyder DS, Fahey JL, Findley DO, Lipsett JA, Zaia JA, Metter GE, Hill LR . Bone marrow transplantation for hematologic malignancies in patients aged 30 years or older J Clin Oncol 1986 4: 1489–1492
Slavin S, Weiss L, Morecki S, Weigensberg M . Eradication of murine leukemia with histoincompatible marrow grafts in mice conditioned with total lymphoid irradiation Cancer Immunother 1981 11: 155–158
Kottaridis PD, Milligan D, Chopra R, Chakraverty R, Chakrabarti S, Robinson S, Peggs K, Verfuerth S, Pettengell R, Marsh JC, Schey MS, Mahendra P, Morgan GJ, Hale G, Waldmann H, Ruiz de Elvira C, Williams CD, Devereux S, Linch D . In vivo CAMPATH-1H prevents graft-versus-host diease following nonmyeloablative stem cell transplantation Blood 2000 96: 2419–2425
Giralt S, Thall P, Khouri I, Wang X, Braunschweig I, Ippolitti C, Claxton D, Donato M, Bruton J, Cohen A, Davis M, Andersson BS, Anderlini P, Gajewski J, Kornblau S, Andreeff M, Przepiorka D, Ueno NT, Molldrem J, Champlin R . Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation Blood 2001 97: 631–637
Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A, Verdonck L, Niederwieser D, van Rhee F, Mittermueller J, de Witte T, Holler E, Anseri H . Graft versus myeloma effect of donor lymphocyte transfusions in marrow grafted patients. European Group for Blood ad Marrow Transplantation Working Party Chronic Leukemia Blood 1995 86: 2041–2050
Kernan NA . T-cell depletion for prevention of graft versus host disease In: Forman SJ, Blume KG, Thomas ED (eds) Bone Marrow Transplantation Blackwell Scientific Publications: Boston, MA 1994 p 124
Gardiner N, Lawler M, O'Riordan J, DeArce M, Humphries P, McCann SR . Persistent donor chimaerism is consistent with disease-free survival following BMT for chronic myeloid leukemia Bone Marrow Transplant 1997 20: 235–241
Wäsch R, Bertz H, Kunzmann R, Finke J . Incidence of mixed chimaerism and clinical outcome in 101 patients after myeloablative conditioning regimens and allogeneic stem cell transplantation Br J Haematol 2000 109: 743–750
McSweeney P, Niederwieser D, Shizuru J, Sandmaier BM, Molina AJ, Maloney DG, Chauncey TR, Gooley TA, Hegenbart U, Nash RA, Radish J, Wagner JL, Minor S, Appelbaum F, Bensinger WI, Bryant E, Flowers ME, Georges GE, Grumet FC, Kiem HP, Torok-Storb B, Yu C, Blume K, Storb R . Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high dose cytotoxic therapy with graft versus tumor effect Blood 2001 97: 3390–3400
Molina A, McSweeny P, Maloney DG, Sandmaier B, Wagner JL, Nash RA, Chauncey T, Bryant E, Storb R . Degree of early donor T-cell chimerism predicts GVHD and graft rejection in patients with non-myeloablative hematopoietic sem cell allografts Blood 1999 94 (Suppl. 1): 1745
Radish J, Gehly G, Lee A, Avery R, Bryant E, Edmands S, Gooley T, Kessler P, Kirk J, Ladne P, Thomas ED, Appelbaum F . Detection of bcr-abl transcripts in Philadelphia chromosome-positive acute lymphoblastic leukemia after marrow transplantation Blood 1997 89: 2602–2609
Radish J, Gehly G, Gooley T, Bryant E, Clift RA, Collins S, Edmands S, Kirk J, Lee A, Kessler P, Schoch G, Buckner CD, Sullivan KM, Appelbaum F, Thomas ED . Polymerase chain reaction detection of the bcr-abl fusion transcript after allogeneic marrow transplantation for chronic myeloid leukemia: results and implications in 346 patients Blood 1995 85: 2632–2638
Childs R, Clave E, Contentin N, Jayasekera D, Hensel N, Leitman S, Read EJ, Carter C, Bahceci E, Young NS, Barret AJ . Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses Blood 1999 94: 3234–3241
Bensinger W, Martin P, Storer B, Clift R, Forman S, Negrin R, Kashyap A, Flowers Mlilleby K, Chauncey T, Storb R, Appelbaum F . Transplantation of bone marrow as compared with peripheral blood cells from HLA identical relatives in patients with hematologic cancers N Engl J Med 2001 344: 175–181
Blaise D, Kuentz M, Fortanier C, Bourhis J, Milpied N, Sutton L, Jouet JP, Attal M, Bordigoni P, Cahn JY, Boiron JM, Schuller MP, Moatti JP, Michallet M . Randomized trial of bone marrow versus lenograstim-primed blood cell allogeneic transplantation in patients with early stage leukemia: a report from the Societe Francaise de Greffe de Moelle J Clin Oncol 2000 18: 537–546
Sullivan K, Agura E, Anasetti C . Chronic graft versus host disease in other late complications of bone marrow transplantation Semin Hematol 1991 28: 250–259
Bader P, Klingebiel T, Schaudt A, Theurer-Mainka U, Handgretinger R, Lang P, Niethammer D, Beck JF . Prevention of relapse in pediatric patients with acute leukemias and MDS after allogeneic SCT by early immunotherapy initiated on the basis of increasing mixed chimerism: a single center experience of 12 children Leukemia 1999 13: 2079–2086
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pérez-Simón, J., Caballero, D., Diez-Campelo, M. et al. Chimerism and minimal residual disease monitoring after reduced intensity conditioning (RIC) allogeneic transplantation. Leukemia 16, 1423–1431 (2002). https://doi.org/10.1038/sj.leu.2402550
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2402550
Keywords
This article is cited by
-
Quantitative chimerism in CD3-negative mononuclear cells predicts prognosis in acute myeloid leukemia patients after hematopoietic stem cell transplantation
Leukemia (2020)
-
Comparison of reduced intensity conditioning regimens used in patients undergoing hematopoietic stem cell transplantation for myelofibrosis
Bone Marrow Transplantation (2019)
-
Adoptive therapy with donor lymphocyte infusion after allogenic hematopoietic SCT in pediatric patients
Bone Marrow Transplantation (2015)
-
Chimerism status is correlated to acute graft-versus-host disease after allogeneic stem cell transplantation
International Journal of Hematology (2014)
-
Combination of fludarabine, amsacrine, and cytarabine followed by reduced-intensity conditioning and allogeneic hematopoietic stem cell transplantation in patients with high-risk acute myeloid leukemia
Annals of Hematology (2013)