Abstract
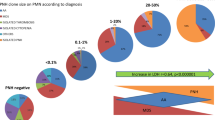
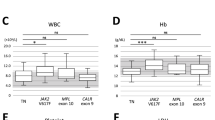
In paroxysmal nocturnal hemoglobinuria (PNH), clonal expansion of glycosylphosphatidylinositol-anchored proteins (GPI-AP)-deficient cells leads to a syndrome characterized by hemolytic anemia, marrow failure, and venous thrombosis. PNH is closely related to aplastic anemia and may share its immune pathophysiology. In vivo expansion of dominant T-cell clones can reflect an antigen-driven immune response but may also represent autonomous proliferation, such as in large granular lymphocytic (LGL)-leukemia. T-cell clonality can be assessed by a combination of T-cell receptor (TCR) flow cytometry and complementarity-determining-region-3 (CDR3) molecular analysis. We studied 24 PNH patients for evidence of in vivo dominant T-cell responses by flow cytometry; TCR-Vβ-specific expansions were identified in all patients. In four cases, extreme expansions of one Vβ-subset of CD8+/CD28-/CD56+ (effector) phenotype mimicked subclinical LGL-disease. The monoclonality of these expansions was inferred from unique CDR3-size peak distributions and sequencing of dominant clonotypes. We conclude that the molecular analysis of TCR-β chain may demonstrate clonal LGL-like expansions at unexpected frequency in PNH patients. Our observations blur the classical boundaries between different bone marrow failure syndromes such as AA, PNH, and LGL, and support the hypothesis that in PNH, the mutant clone may expand as a result of an immune-escape from antigen-driven lymphocyte attack on hematopoietic progenitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Takeda J, Miyata T, Kawagoe K, Iida Y, Endo Y, Fujita T et al. Dificiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell 1993; 73: 703–711.
Luzzatto L, Bessler M, Rotoli B . Somatic mutations in paroxysmal nocturnal hemoglobinuria: a blessing in disguise. Cell 1997; 88: 1–4.
Young NS, Maciejewski JP . Genetic and environmental effects in paroxysmal nocturnal hemoglobinuria: this little PIG-A goes ‘Why? Why? Why?’. J Clin Invest 2000; 106: 637–641.
Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV . Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med 1995; 333: 1253–1258.
Maciejewski JP, Follman D, Rivera CE, Brown K, Simonis T, Young NS . Increased frequency of HLA-DR2 in patients with paroxysmal nocturnal hemoglobinuria and PNH/aplastic anemia syndrome. Blood 2001; 98: 3513–3519.
Karadimitris A, Manavian JS, Thaler HT, Notaro R, Araten DJ, Nafa K et al. Abnormal T-cell repertoire is consistent with immune process underlying the pathogenesis of paroxysmal nocturnal hemoglobinuria. Blood 2000; 96: 2613–2620.
Risitano AM, Kook H, Zeng W, Chen G, Young NS, Maciejewski JP . Oligoclonal and polyclonal CD4 and CD8 lymphocytes in aplastic anemia and paroxysmal nocturnal hemoglobinuria measured by Vβ CDR3 spectratyping and flow cytometry. Blood 2002; 100: 178–183.
Kronenberg M, Siu G, Hood LE, Shastri N . The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol 1996; 4: 529–591.
Plasilova M, Risitano A, Maciejewski JP . Application of the molecular analysis of the T-cell receptor repertoire in the study of immune-mediated hematologic diseases. Hematology 2003; 8: 173–181.
van den Beemd R, Boor PP, van Lochem EG, Hop WC, Langerak AW, Wolvers-Tettero IL et al. Flow cytometric analysis of the Vbeta repertoire in healthy controls. Cytometry 2000; 40: 336–345.
Pannetier C, Even J, Kourilsky P . T cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today 1995; 16: 176–181.
Even J, Lim A, Puisieux I, Ferradini L, Dietrich PY, Toubert A et al. T-cell repertoires in healthy and diseased human tissues analysed by T-cell receptor β-chain CDR3 size determination: evidence for oligoclonal expansions in tumours and inflammatory diseases. Res Immunol 1995; 146: 65–80.
Risitano AM, Maciejewski JP, Green S, Plasilova M, Weng Z, Young NS . In vivo dominant immune responses in aplastic anemia patients: molecular tracking of putatively pathogenic T cell clones by TCR-β-CDR3 sequencing. Lancet 2004; 364: 355–364.
Plasilova M, Risitano AM, O'Keefe CL, Rodriguez R, Wlodarski M, Young NS et al. Shared and individual specificities of immunodominant cytotoxic T cell clones in paroxysmal nocturnal hemoglobinuria as determined by molecular analysis. Exp Hematol 2004; 32: 261–269.
Kochenderfer JN, Kobayashi S, Wieder ED, Su C, Molldrem JJ . Loss of T-lymphocyte clonal dominance in patients with myelodysplastic syndrome responsive to immunosuppression. Blood 2002; 100: 3639–3645.
Lamy T, Loughran Jr TP . Clinical features of large granular lymphocytic leukemia. Semin Hematol 2003; 40: 185–195.
O'Keefe C, Plasilova M, Risitano A, Rodriguez A, Wlodarski M, Young NS et al. Molecular analysis of TCR clonotypes in LGL: a clonal model for polyclonal responses. J Immunol 2004; 172: 1960–1969.
Karadimitris A, Li K, Notaro R, Araten DJ, Nafa K, Thertulien R et al. Association of clonal T-cell large granular lymphocyte disease and paroxysmal nocturnal haemoglobinuria (PNH): further evidence for a pathogenetic link between T cells, aplastic anaemia and PNH. Br J Haematol 2001; 115: 1010–1014.
Dunn DE, Tannawattanacharoen P, Boccuni P, Nagakura S, Green SW, Kirby MR et al. Paroxysmal nocturnal hemoglobinuria cells in patients with bone marrow failure syndromes. Ann Intern Med 1999; 131: 401–408.
International Agranulocytosis and Aplastic Anemia Study. Incidence of aplastic anemia: the relevance of diagnostic criteria. Blood 1987; 70: 1718–1723.
Zeng W, Maciejewski JP, Chen G, Young NS . Limited heterogeneity of T-cell receptor VB usage in aplastic anemia. J Clin Invest 2001; 108: 765–773.
Semenzato G, Zambello R, Starkebaum G, Oshimi K, Loughran Jr TP . The lymphoproliferative disease of granular lymphocytes: updated criteria for diagnosis. Blood 1997; 89: 256–260.
Kanchan K, Loughran Jr TP . Antigen-driven clonal T cell expansion in disorders of hematopoiesis. Leuk Res 2003; 27: 291–292.
Epling-Brunette PK, Loughran Jr TP . Survival signals in leukemic large granular lymphocytes. Semin Hematol 2003; 40: 213–220.
McHeyzer-Williams LJ, Panus JF, Mikszta JA, McHeyzer-Williams MG . Evolution of antigen-specific T cell receptors in vivo: preimmune and antigen-driven selection of preferred complementarity-determining region 3 (CDR3) motifs. J Exp Med 1999; 189: 1823–1838.
Akashi K, Shibuya T, Taniguchi S, Hayashi S, Iwasaki H, Teshima T et al. Multiple autoimmune haemopoietic disorders and insidious clonal proliferation of large granular lymphocytes. Br J Haematol 1999; 107: 670–673.
Bowman SJ, Bhavnani M, Geddes GC, Corrigall V, Boylston AW, Panayi GS et al. Large granular lymphocyte expansions in patients with Felty's syndrome: analysis using anti-T cell receptor V beta-specific monoclonal antibodies. Clin Exp Immunol 1995; 101: 18–24.
Kook H, Zeng W, Guibin C, Kirby M, Young NS, Maciejewski JP . Increased cytotoxic T cells with effector phenotype in aplastic anemia and myelodysplasia. Exp Hematol 2001; 29: 1270–1277.
Young NS, Maciejewski J . The pathophysiology of acquired aplastic anemia. N Engl J Med 1997; 336: 1365–1372.
Young NS . Hematopoietic cell destruction by immune mechanisms in aquired aplastic anemia. Semin Hematol 2000; 37: 3–14.
Maciejewski JP, Rivera C, Kook H, Dunn D, Young NS . Relationship between bone marrow failure syndromes and the presence of glycophosphatidyl inositol-anchored protein-deficient clones. Br J Haematol 2001; 115: 1015–1022.
Wang H, Chuhjo T, Yasue S, Omine M, Nakao S . Clinical significance of a minor population of paroxysmal nocturnal hemoglobinuria-type cells in bone marrow failure syndrome. Blood 2002; 100: 3897–3902.
Murakami Y, Kosaka H, Maeda Y, Nishimura J, Inoue N, Ohishi K et al. Ineffecient response of T lymphocytes to glycosylphosphatidylinositol anchor-negative cells: implications for paroxysmal nocturnal hemoglobinuria. Blood 2002; 100: 4116–4122.
Nagakura S, Ishihara S, Dunn DE, Nishimura J, Kawaguchi T, Horikawa K et al. Decreased susceptibility of leukemic cells with PIG-A mutation to natural killer cells in vitro. Blood 2002; 100: 1031–1037.
Kai T, Shichishima T, Noji H, Yamamoto T, Okamoto M, Ikeda K et al. Phenotypes and phosphatidylinositol glycan-class A gene abnormalities during cell differentiation and maturation from precursor cells to mature granulocytes in patients with paroxysmal nocturnal hemoglobinuria. Blood 2002; 100: 3812–3818.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Risitano, A., Maciejewski, J., Muranski, P. et al. Large granular lymphocyte (LGL)-like clonal expansions in paroxysmal nocturnal hemoglobinuria (PNH) patients. Leukemia 19, 217–222 (2005). https://doi.org/10.1038/sj.leu.2403617
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403617
Keywords
This article is cited by
-
All that glitters is not LGL Leukemia
Leukemia (2022)
-
T-cell clones of uncertain significance are highly prevalent and show close resemblance to T-cell large granular lymphocytic leukemia. Implications for laboratory diagnostics
Modern Pathology (2020)
-
Large granular lymphocytic leukemia coexists with myeloid clones and myelodysplastic syndrome
Leukemia (2020)
-
Features, reason for testing, and changes with time of 583 paroxysmal nocturnal hemoglobinuria clones from 529 patients: a multicenter Italian study
Annals of Hematology (2019)
-
Paroxysmal nocturnal haemoglobinuria
Nature Reviews Disease Primers (2017)