Abstract
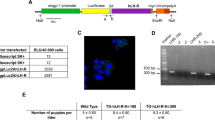
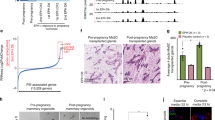
H19 is an imprinted and developmentally regulated gene whose product remains apparently untranslated. In a previous study on breast adenocarcinomas, we reported that overexpression of the H19 gene was significantly correlated with the presence of steroid receptors, suggesting the putative role of hormones in H19 transcription. To determine the mode of steroid action, we have detected levels of H19 RNA synthesis during mammary gland development by in situ hybridization (ISH): two peaks of H19 transcription occur during puberty and pregnancy. Furthermore, we demonstrated by ISH that in the uterus H19 RNA synthesis is high during estrus and metestrus phases. To test steroid control of H19 transcription, ovariectomized and adrenalectomized mice were supplemented, 1 week after surgery, with 17-β-estradiol (E2, 20 μg/kg/day), progesterone (P, 1 mg/kg/day) or corticosterone (B, 0.3 mg/kg/day) for 2 weeks. According to ISH data, E2 and to a lesser extent B stimulated H19 transcription in the uterus, whereas P inhibited it. To confirm the in vivo results, in vitro experiments were performed using cultures of MCF-7 cells (a hormone-sensitive mammary cell line). E2 stimulated the endogenous H19 gene of this cell line and tamoxifen inhibited this effect. Furthermore, we performed transient cotransfections in MCF-7, in HBL-100 (another hormone-sensitive mammary cell line) and in BT-20 (a hormone-insensitive mammary cell line) with various constructs of ERα (WT or mutated) and PR-A, in presence or absence of steroid hormones. We demonstrated that ERα up-regulated the H19 promoter in MCF-7 and in HBL-100, whereas PR-A did not have any effect per se. Moreover, in MCF-7, PR-A antagonized clearly the ERα-mediated promoter enhancement, but in HBL-100 this counteracting effect on the ERα up-regulation was not found. Interestingly, the same experiments performed in BT-20 cell line provided very similar results as those obtained in MCF-7 cells, with a clear down-regulation mediated by PR-A on the H19 promoter. All these in vitro data are in agreement with in vivo results. In addition, data obtained with ERα mutants indicate that H19 promoter activation is both ligand-dependent and ligand-independent. We have thus demonstrated that H19 gene expression is controlled by steroid hormones; furthermore, this gene is highly expressed in hormone-sensitive organs when the hormonal stimulation is accompanied with a morphological repair.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Adriaenssens E, Dumont L, Lottin S, Bolle D, Leprêtre A, Delobelle A, Bouali F, Dugimont T, Coll J and Curgy JJ. . 1998 Am. J. Pathol. 153: 1597–1607.
Ariel I, Lustig O, Oyer CE, Elkin M, Gonik J, Rachmilewitz J, Biran H, Goshen R, de Groot N and Hochberg A. . 1994 Gynecol. Oncol. 53: 212–219.
Ariel I, Ayesh S, Perlman EJ, Pizov G, Tanos V, Schneider T, Erdmann VA, Podeh D, Komitowski D, Quasem AS, de Groot N and Hochberg A. . 1997a Mol. Pathol. 50: 34–44.
Ariel I, Weinstein D, Voutilainen R, Schneider T, Lustig-Yariv O, de Groot N and Hochberg A. . 1997b Diagn. Mol. Pathol. 6: 17–25.
Aumais JP, Lee HS, DeGannes C, Horsford J and White HJ. . 1996 J. Biol. Chem. 271: 12568–12577.
Bartolomei MS, Zemel S and Tilghman SM. . 1991 Nature 351: 153–155.
Bartolomei MS, Webber AL, Brunkow ME and Tilghman SM. . 1993 Genes Dev. 7: 1663–1673.
Bernet F, Maubert E, Bernard J, Montel V and Dupouy JP. . 1994 Regul. Peptides 52: 187–193.
Biran H, Ariel I, de Groot N, Shani A and Hochberg A. . 1994 Tumour Biol. 15: 123–134.
Brannan CI, Dees EC, Ingram RS and Tilghman SM. . 1990 Mol. Cell. Biol. 10: 28–36.
Chatelain A, Durand P, Naaman E and Dupouy JP. . 1989 J. Endocrinol. 123: 421–428.
Cooper MJ, Fischer M, Komitowski D, Shevelev A, Schulze E, Ariel I, Tykocinski ML, Miron S, Ilan J, de Groot N and Hochberg A. . 1996 J. Urol. 155: 2120–2127.
Cui H-M, Hedborg F, He L, Nordenskjöld A, Sandstedt B, Pfeifer-Ohlsson S and Ohlsson R. . 1997 Cancer Res. 57: 4469–4473.
Cunha GR, Young P, Hamamoto S, Guzman R and Nandi S. . 1992 Epith. Cell Biol. 1: 105–118.
Dalle M, Giry J, Gay M and Delost T. . 1978 J. Endocrinol. 76: 303–309.
Daniel CW and Robinson SD. . 1992 Mol. Reprod. Dev. 32: 145–151.
DeChiara TM, Robertson EJ and Efstratiadis A. . 1991 Cell 64: 849–859.
Douc-Rasy S, Coll J, Barrois M, Joubel A, Prost S, Dozier C, Stéhelin D and Riou G. . 1993 Int. J. Oncol. 2: 753–758.
Dugimont T, Curgy JJ, Wernert N, Delobelle A, Raes MB, Joubel A, Stéhelin D and Coll J. . 1995 Biol. Cell 85: 117–124.
Dugimont T, Montpellier C, Adriaenssens E, Lottin S, Dumont L, Iotsova V, Lagrou C, Stéhelin D, Coll J and Curgy JJ. . 1998 Oncogene 16: 2395–2401.
Dupouy JP, Coffigny M and Magre S. . 1975 Endocrinol. 65: 347–352.
Dupouy JP and Cohen A. . 1975 C. R. Acad. Sci. Paris 280: 463–466.
El Tanani MK and Green CD. . 1997 Mol. Endocrinol. 11: 928–937.
El Yazidi I, Renaud F, Laurent M, Courtois Y and Boilly-Marer Y. . 1998 Biochim. Biophys. Acta 1403: 127–140.
Eversole-Cire P, Fergusson-Smith AC, Surani MA and Jones PA. . 1995 Cell Growth Differ. 6: 337–345.
Giangrande PH, Pollio G and McDonnell DP. . 1997 J. Biol. Chem. 272: 32889–32900.
Glaser T, Housman D, Lewis WH, Gerhard D and Jones C. . 1989 Somat. Cell. Mol. Genet. 15: 477–501.
Gronemeyer H, Meyer M-E, Bocquell M-T, Kastner P, Turcotte B and Chambon P. . 1991 Steroid Biochem. Mol. Biol. 40: 271–278.
Guiochon-Mantel A, Loosfelt H, Ragot T, Bailly A, Atger M, Misrahi M, Perricaudet M and Milgrom E. . 1988 Nature 336: 695–698.
Han DKM, Khaing ZZ, Pollock RA, Haudenschild CC and Liau G. . 1996 J. Clin. Invest. 97: 1276–1285.
Hanstein B, Liu H, Yancisin MC and Brown M. . 1999 Molec. Endocrinol. 13: 129–137.
Hao Y, Crenshaw T, Moulton T, Newcomb E and Tycko B. . 1993 Nature 365: 764–767.
Haslam SZ and Shyamala G. . 1980 J. Cell Biol. 86: 730–737.
Haslam SZ. . 1987. In: The Mammary Gland: Development, Regulation and function. Neville MC and Daniel CW (eds). . New York: Plenum Press. pp. 499–533.
Hemberger M, Redies C, Krause R, Oswald J, Walter J and Fundele RH. . 1998 Dev. Genes Evol. 208: 393–402.
Ilenchuck TT and Walters MR. . 1987 Endocrinology 120: 1449–1456.
Ince BA, Schodin DJ, Shapiro DJ and Katzenellenbogen BS. . 1995 Endocrinology 136: 3194–3199.
Jones CM, Lyons KM and Hogan BLM. . 1991 Development 111: 531–542.
Joubel A, Curgy JJ, Pelczar H, Begue A, Lagrou C, Stéhelin D and Coll J. . 1996 Cell. Mol. Biol. 42: 1159–1172.
Kato S, Sasaki H, Suzawa M, Masushige S, Tora L, Chambon P and Gronemeyer H. . 1995 Mol. Cell. Biol. 15: 5858–5867.
Kondo M and Takahashi T. . 1996 Nippon Rinsho 54: 492–496.
Kopf E, Bibi O, Ayesh S, Tykocinski M, Vitner K, Looijenga LHJ, de Groot N and Hochberg A. . 1998 FEBS Lett. 432: 123–127.
Kraus WL, Weis KE and Katzenellengogen BS. . 1995 Molec. Cell. Biol. 15: 1847–1857.
Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S and Gustfsson J-A. . 1996 Proc. Natl. Acad. Sci USA 93: 5925–5930.
Lahooti H, White R, Hoare SA, Rahman D, Pappin DJ and Parker MG. . 1995 J. Steroid. Biochem. Mol. Biol. 55: 305–313.
Lee AV and Yee D. . 1995 Biomed. Pharmacother. 49: 415–421.
Leibovitch MP, Nguyen VC, Gross MS, Solhonne B, Leibovitch SA and Berheim A. . 1991 Biochem. Biophys. Res. Commun. 180: 1241–1250.
Leighton PA, Saam JR, Ingram RS, Stewart CL and Tilghman SM. . 1995 Genes Dev. 9: 2079–2089.
Lesage J, Bernet F, Montel V and Dupouy JP. . 1996 Neurochem. Res. 21: 87–96.
Li Y-M, Franklin G, Cui H-M, Svensson K, He X-B, Adam G, Ohlsson R and Pfeifer S. . 1998 J. Biol. Chem. 273: 28247–28252.
Lustig-Yariv O, Schulze E, Komitowski D, Erdmann V, Schneider T, de Groot N and Hochberg A. . 1997 Oncogene 15: 169–177.
Marie J, Legrand C and Maltier JP. . 1980 Pathol. Biol. 28: 377–379.
Marie J, Legrand C, Maltier JP and Scholler R. . 1981 Pathol. Biol. 29: 367–370.
McCormack JT and Greenwald GS. . 1974 J. Endocrinol. 62: 101–107.
Mosselman S, Polman J and Dijkema R. . 1996 FEBS Let. 392: 49–53.
Moulton T, Crenshaw T, Hao Y, Moosikawuwan J, Lin N, Dembitzer F, Hensle T, Weiss L, McMorrow L, Loew T, Kraus W, Gerald W and Tycko B. . 1994 Nature Genet. 7: 440–447.
Nandi S. . 1958 J. Natl. Cancer Inst. 21: 1039–1063.
Pachnis V, Belayew A and Tilghman SM. . 1984 Proc. Natl. Acad. Sci. USA 81: 5523–5527.
Pachnis V, Brannan CI and Tilghman SM. . 1988 Mol. Cell. Biol. 8: 4707–4715.
Quéva C, Ness SA, Grässer FA, Graf T, Vandenbunder B and Stéhelin D. . 1992 Development 114: 125–133.
Peyrat J-P, Hondermark H, Louchez M-M and Boilly B. . 1991 Cancer Com. 3: 323–329.
Reese JC and Katzenellenbogen BS. . 1991 J. Biol. Chem. 266: 10880–10887.
Rhoda J, Corbier P and Roffi J. . 1984 Endocrinol. 114: 1754–1760.
Rhoda J, Corbier P and Roffi J, Castanier M and Scholler R. . 1983 C. R. Acad. Sci. Paris 296: 405–408.
Sakakura T. . 1991 Int. Rev. Cytol. 125: 165–201.
Sambrook J, Fritsch EF and Maniatis T. . 1989 Cold Spring Harbor Laboratory Press: 2nd Edn. CSH Press: Plainview, New York, pp. 7.19–7.22.
Sanyal MK. . 1978 J. Endocrinol. 79: 179–190.
Schodin DJ, Zhuang Y, Shapiro DJ and Katzenellenbogen BS. . 1995 J. Biol. Chem 270: 31163–31171.
Smith MS, Freeman ME and Neill JD. . 1975 Endocrinol. 96: 219–226.
Snedeker SM, Brown CF and DiAugustine RP. . 1991 Proc. Natl. Acad. Sci. USA 88: 276–280.
Taniguchi T, Sullivan MJ, Ogawa O and Reeve AE. . 1995 Proc. Natl. Acad. Sci. USA 92: 2159–2163.
Thesleff I, Vaahtokari A and Partanen AM. . 1995 Int. J. Dev. Biol. 39: 35–50.
Topper YJ and Freeman CS. . 1980 Physiol. Rev. 60: 1049–1106.
Van Roozendaal CEP, Gillis AJM, Klijn JGM, Van Ooijen B, Claasen CJC, Eggermont AMM, Henzen-Logmans SC, Oosterhuis JW, Foekens JA and Looijenga LHJ. . 1998 FEBS Let. 437: 107–111.
Voutilainen R, Ilvesmaki V, Ariel I, Rachmilewitz J, de Groot N and Hochberg A. . 1994 Endocrinology 134: 2051–2056.
Wang WH, Duan JX, Vu TH and Hoffman AR. . 1996 J. Biol. Chem. 271: 27863–27870.
Webber AL, Ingram RS, Levorse JM and Tilghman SM. . 1998 Nature 391: 711–715.
Weigel NL and Zhang Y. . 1998 J. Mol. Med. 76: 469–479.
Weisz J and Ward IL. . 1980 Endocrinol. 106: 306–316.
Yballe CM, Vu TH and Hoffman AR. . 1996 J. Clin. Endocrinol. Metab. 81: 1607–1612.
Yoo-Warren H, Pachnis V, Ingram RS and Tilghman SM. . 1988 Mol. Cell Biol. 8: 4707–4715.
Zemel S, Barolomei MS and Tilghman SM. . 1992 Nat. Genet. 2: 61–65.
Zhang Y and Tycko B. . 1992 Nat. Genet. 1: 40–44.
Acknowledgements
We would like to thank Dr Anne Chauchereau (INSERM U135, University Paris-Sud, Le Kremlin-Bicêtre), Dr Gwendal Lazennec (Montpellier) and Dr Franck Delaunay for graciously providing wild-type progesterone, wild-type and E domain mutated estrogen receptors, AF-1 mutated ones, respectively. We thank also Dr Brigitte Fournier (Novartis, Bale) for her generous gift of the ERE-Luc vector. Thanks to Dr Julie Kerr-Conte (Lille) and Dr Victor Nurcombe (University of Queensland, St. Lucia, Brisbane, Australia) for critical reading of the manuscript. We are indebted to Dr Bernard Vandenbunder (Institut de Biologie Moléculaire, Lille) for our training in his laboratory and to Fatima Bouali for her help in in situ hybridization technique. We are grateful to Chantal Pennel (Centre anti-cancéreux Oscar Lambret, Lille) and Isabelle Pollet for their help in histological methods. This work was supported by grants from Pasteur Institute of Lille. Jean-Jacques Curgy holds grants from the Fédération des Groupements des Entreprises Françaises dans la Lutte contre le Cancer (FéGEFLUC), from the Comité Nord de la Ligue Nationale contre le Cancer and from the NORGINE PHARMA laboratories (Paris). Eric Adriaenssens is a recipient of an ARC (Association pour la Recherche sur le Cancer) fellowship.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Adriaenssens, E., Lottin, S., Dugimont, T. et al. Steroid hormones modulate H19 gene expression in both mammary gland and uterus. Oncogene 18, 4460–4473 (1999). https://doi.org/10.1038/sj.onc.1202819
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1202819
Keywords
This article is cited by
-
Tamoxifen exerts anti-peritoneal fibrosis effects by inhibiting H19-activated VEGFA transcription
Journal of Translational Medicine (2023)
-
The role of long non-coding RNA H19 in infertility
Cell Death Discovery (2023)
-
Expression and diagnostic values of MIAT, H19, and NRON long non-coding RNAs in multiple sclerosis patients
Egyptian Journal of Medical Human Genetics (2022)
-
The pro-oncogenic effect of the lncRNA H19 in the development of chronic inflammation-mediated hepatocellular carcinoma
Oncogene (2021)
-
L-lactate induces specific genome wide alterations of gene expression in cultured bovine granulosa cells
BMC Genomics (2019)