Abstract
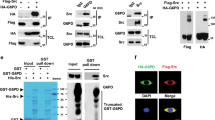
RACK1 is one of a group of PKC-interacting proteins collectively called RACKs (Receptors for Activated C-Kinases). Previously, we showed that RACK1 also interacts with the Src tyrosine kinase, and is an inhibitor of Src activity and cell growth. PKC activation induces the intracellular movement and co-localization of RACK1 and Src, and the tyrosine phosphorylation of RACK1. To determine whether RACK1 is a Src substrate, we assessed phosphorylation of RACK1 by various tyrosine kinases in vitro, and by kinase-active and inactive mutants of Src in vivo. We found that RACK1 is a Src substrate. Moreover, Src activity is necessary for both the tyrosine phosphorylation of RACK1 and the binding of RACK1 to Src's SH2 domain that occur following PKC activation. To identify the tyrosine(s) on RACK1 that is phosphorylated by Src, we generated and tested a series of RACK1 mutants. We found that Src phosphorylates RACK1 on Tyr 228 and/or Tyr 246, highly-conserved tyrosines located in the sixth WD repeat that interact with Src's SH2 domain. We think that RACK1 is an important Src substrate that signals downstream of growth factor receptor tyrosine kinases and is involved in the regulation of Src function and cell growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Abram CL, Courtneidge SA . 2000 Exp. Cell. Res. 254: 1–13
Bjorge JD, Jakymiw A, Fujita DJ . 2000 Oncogene 19: 5620–5635
Cartwright CA, Eckhart W, Simon S, Kaplan PL . 1987 Cell 49: 83–91
Cartwright CA, Hutchinson M, Eckhart W . 1985 Mol. Cell. Biol. 5: 2647–2652
Cartwright CA, Kaplan PL, Cooper JA, Hunter T, Eckhart W . 1986 Mol. Cell. Biol. 6: 1562–1570
Cartwright CA, Meisler MA, Eckhart W . 1990 Proc. Natl. Acad. Sci. USA 87: 558–562
Chang BY, Chiang M, Cartwright CA . 2001 J. Biol. Chem. 276: 20346–20356
Chang BY, Conroy KB, Machleder EM, Cartwright CA . 1998 Mol. Cell. Biol. 18: 3245–3256
Chen RH, Miettinen PJ, Maruoka EM, Choy L, Derynck R . 1995 Nature 377: 548–552
Glenny JR, Zokas L, Kamps MP . 1988 J. Imm. Meth. 109: 277–285
Kmiecik TE, Shalloway D . 1987 Cell 49: 65–73
Lipsich LA, Lewis AJ, Brugge JS . 1983 J. Virol. 48: 352–360
Lou Q, Leftwich ME, Lam KS . 1996 Bioorg. Med. Chem. 4: 677–682
Martin GS . 2001 Nature Rev. Mol. Cell Biol. 2: 467–475
Mayer BJ, Hirai H, Sakai R . 1995 Curr. Biol. 5: 296–305
Mochly-Rosen D, Kauvar LM . 1998 Adv. Pharm. 44: 91–145
Mochly-Rosen D . 1995 Science 268: 247–251
Mourton T, Hellberg CB, Burden-Gulley SM, Hinman J, Rhee A, Brady-Kalnay SM . 2001 J. Biol. Chem. 276: 14896–14901
Moyers JS, Bouton AB, Parsons SJ . 1993 Mol. Cell. Biol. 13: 2391–2400
Neer EJ, Smith TF . 1996 Cell 84: 175–178
Neer EJ, Schmidt CJ, Nambudripad R, Smith TF . 1994 Nature 371: 297–300
Park J, Cartwright CA . 1995 Mol. Cell. Biol. 15: 2374–2382
Pellicena P, Stowell KR, Miller WT . 1998 J. Biol. Chem. 273: 15325–15328
Piwnica-Worms H, Saunders KB, Roberts TM, Smith AE, Cheng SH . 1987 Cell 49: 75–82
Reynolds AB, Vila J, Lansing TJ, Potts WM, Weber MJ, Parsons JT . 1987 EMBO J. 6: 2359–2364
Schechtman D, Mochly-Rosen D . 2001 Oncogene 20: 6339–6347
Schmitz R, Baumann G, Gram H . 1996 J. Mol. Biol. 260: 664–677
Snyder MA, Bishop JM, McGrath JP, Levinson AD . 1985 Mol. Cell. Biol. 5: 1772–1779
Thomas SM, Brugge JA . 1997 Annu. Rev. Cell Dev. Biol. 13: 513–609
Zhou S, Caraway III KL, Eck MJ, Harrison SC, Feldman RA, Mohammadl M, Schlessinger J, Hubbard SR, Smith DP, Eng C, Lorenzo ML, Ponder BAJ, Mayer BJ, Cantley LC . 1995 Nature 373: 536–544
Acknowledgements
We thank David Shalloway for NIH3T3 cell lines expressing v-Src or c-Src mutants Y527F, Y527F/Y416F or Y416F. We are grateful to Jean Wang for purified Abl kinase, Sarah Parsons for pMdl155c-src and Tony Hunter for pGEM K297M. We thank Daria Mochly-Rosen, Anson Lowe and Bishr Omary for critical review of the data. We are grateful to Blanca Pineda for assistance with preparation of the manuscript and figures. This work was supported by grants from the National Institutes of Health to CA Cartwright (R01 DK43743) and to BY Chang (National Research Service Award CA69810).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, B., Harte, R. & Cartwright, C. RACK1: a novel substrate for the Src protein-tyrosine kinase. Oncogene 21, 7619–7629 (2002). https://doi.org/10.1038/sj.onc.1206002
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206002
Keywords
This article is cited by
-
Skin chronological aging drives age-related bone loss via secretion of cystatin-A
Nature Aging (2022)
-
Dual functions of Rack1 in regulating Hedgehog pathway
Cell Death & Differentiation (2020)
-
Rack1 mediates Src binding to drug transporter P-glycoprotein and modulates its activity through regulating Caveolin-1 phosphorylation in breast cancer cells
Cell Death & Disease (2019)
-
RACK1 interaction with c-Src is essential for osteoclast function
Experimental & Molecular Medicine (2019)
-
Crystal structure of Gib2, a signal-transducing protein scaffold associated with ribosomes in Cryptococcus neoformans
Scientific Reports (2015)