Abstract
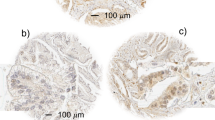
The novel mitogen/extracellular-signal-regulated kinase kinase 5/extracellular signal-regulated kinase-5 (MEK5/ERK5) pathway has been implicated in the regulation of cellular proliferation. MEK5 expression has been detected in prostate cancer cells, although the significance of the MEK5/ERK5 pathway in human prostate cancer has not been tested. We examined MEK5 expression in 127 cases of prostate cancer and 20 cases of benign prostatic hypertrophy (BPH) by immunohistochemistry and compared the results to clinical parameters. We demonstrated that MEK5 expression is increased in prostate cancer as compared to benign prostatic tissue. Strong MEK5 expression correlates with the presence of bony metastases and less favourable disease-specific survival. Furthermore, among the patients with high Gleason score of 8–10, MEK5 overexpression has an additional prognostic value in survival. MEK5 transfection experiments confirm its ability to induce proliferation (P<0.0001), motility (P=0.0001) and invasion in prostate cancer cells (P=0.0001). MEK5 expression drastically increased MMP-9, but not MMP-2 mRNA expression. Luciferase report assays suggest that the −670/MMP-9 promoter is upregulated by MEK5 and electromobility shift assay further suggests the involvement of activator protein-I (AP-1), but not the NF-κB, binding site in the MMP-9 promoter. Using an AP-1 luciferase construct, activation of MEK5 was confirmed to enhance AP-1 activities up to twofold. Taken together, our results establish MEK5 as a key signalling molecule associated with prostate carcinogenesis. As the MEK5/ERK5 interaction is highly specific, it represents a potential target of therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Abe J, Kusuhara M, Ulevitch RJ, Berk BC and Lee JD . (1996). J. Biol. Chem., 271, 16586–16590.
Adler HL, McCurdy MA, Kattan MW, Timme TL, Scardino PT and Thompson TC . (1999). J. Urol., 161, 182–187.
Autzen P, Robson CN, Bjartell A, Malcolm AJ, Johnson MI, Neal DE and Hamdy FC . (1998). Br. J. Cancer, 78, 1219–1223.
Dong Z, Nemeth JA, Cher ML, Palmer KC, Bright RC and Fridman R . (2001). Int. J. Cancer, 93, 507–515.
Eastham JA, Truong LD, Rogers E, Kattan M, Flanders KC, Scardino PT and Thompson TC . (1995). Lab. Invest., 73, 628–635.
English JM, Vanderbilt CA, Xu S, Marcus S and Cobb MH . (1995). J. Biol. Chem., 270, 28897–28902.
Esparis-Ogando A, Diaz-Rodriguez E, Montero JC, Yuste L, Crespo P and Pandiella A (2002). Mol. Cell Biol., 22, 270–285.
Foda HD and Zucker S . (2001). Drug Discov. Today, 6, 478–482.
Greenlee RT, Hill-Harmon MB, Murray T and Thun M . (2001). CA Cancer J. Clin., 51, 15–36.
Hamdy FC, Autzen P, Robinson MC, Horne CH, Neal DE and Robson CN . (1997). Cancer Res., 57, 4427–4431.
Hamdy FC, Fadlon EJ, Cottam D, Lawry J, Thurrell W, Silcocks PB, Anderson JB, Williams JL and Rees RC . (1994). Br. J. Cancer, 69, 177–182.
Hayakawa J, Ohmichi M, Kurachi H, Ikegami H, Kimura A, Matsuoka T, Jikihara H, Mercola D and Murata Y . (1999). J. Biol. Chem., 274, 31648–31654.
Hetman M, Kanning K, Cavanaugh JE and Xia Z . (1999). J. Biol. Chem., 274, 22569–22580.
Jenkins BL, Mehta PB, Robson CN, Neal DE and Leung HY . (2001). Br. J. Cancer, 85(Suppl 1), 22.
Kamakura S, Moriguchi T and Nishida E . (1999). J. Biol. Chem., 274, 26563–26571.
Kato Y, Kravchenko VV, Tapping RI, Han J, Ulevitch RJ and Lee JD . (1997). EMBO J., 16, 7054–7066.
Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ and Lee JD . (1998). Nature, 395, 713–716.
Kostenuik PJ, Singh G, Suyama KL and Orr FW . (1992). Clin. Exp. Metast., 10, 411–418.
McCawley LJ, Li S, Wattenberg EV and Hudson LG . (1999). J. Biol. Chem., 274, 4347–4353.
McCawley LJ and Matrisian LM . (2000). Mol. Med. Today, 6, 149–156.
Okamoto M, Lee C and Oyasu R . (1997). Cancer Res., 57, 141–146.
Pearson G, English JM, White MA and Cobb MH . (2001). J. Biol. Chem., 276, 7927–7931.
Price DT, Rocca GD, Guo C, Ballo MS, Schwinn DA and Luttrell LM . (1999). J. Urol., 162, 1537–1542.
Ricca A, Biroccio A, Del Bufalo D, Mackay AR, Santoni A and Cippitelli M . (2000). Int. J. Cancer, 86, 188–196.
Robinson D, He F, Pretlow T and Kung HJ . (1996). Proc. Natl. Acad. Sci. USA, 93, 5958–5962.
Sato H and Seiki M . (1993). Oncogene, 8, 395–405.
Scorilas A, Karameris A, Arnogiannaki N, Ardavanis A, Bassilopoulos P, Trangas T and Talieri M . (2001). Br. J. Cancer, 84, 1488–1496.
Sebolt-Leopold JS, Dudley DT, Herrera R, Van Becelaere K, Wiland A, Gowan RC, Tecle H, Barrett SD, Bridges A, Przybranowski S, Leopold WR and Saltiel AR . (1999). Nat. Med., 5, 810–816.
Sehgal G, Hua J, Bernhard EJ, Sehgal I, Thompson TC and Muschel RJ . (1998). Am. J. Pathol., 152, 591–596.
Stack MS, Ellerbroek SM and Fishman DA . (1998). Int. J. Oncol., 12, 569–576.
Stadheim TA, Saluta GR and Kucera GL . (2000). Biochem. Pharmacol., 59, 407–418.
Stearns ME and Wang M . (1993). Cancer Res., 53, 878–883.
Stone AA and Chambers TC . (2000). Exp. Cell Res., 254, 110–119.
Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ and Gillespie MT . (1999). Endocrinology, 140, 4451–4458.
Troussard AA, Costello P, Yoganathan TN, Kumagai S, Roskelley CD and Dedhar S . (2000). Oncogene, 19, 5444–5452.
Wood M, Fudge K, Mohler JL, Frost AR, Garcia F, Wang M and Stearns ME . (1997). Clin. Exp. Metast., 15, 246–258.
Yan C, Wang H and Boyd DD . (2001). J. Biol. Chem., 276, 1164–1172.
Zhou G, Bao ZQ and Dixon JE . (1995). J. Biol. Chem., 270, 12665–12669.
Acknowledgements
We acknowledge the following for their help: Dr JD Lee (Department of Immunology, The Scripps Research Institute, USA) for the ERK5 antibody and CMV-MEK5D construct; Dr Y Sun (Parke Davies Pharmaceutical Research, UK) for the MMP-2 promoter construct; Dr D Boyd (M. D. Anderson Cancer Centre, USA) for the MMP-9 promoter constructs; and to Dr A Pulimood (University of Newcastle, UK) for assistance with histopathological grading. This project was supported by grants from the Newcastle Hospitals Trustees and British Urological Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehta, P., Jenkins, B., McCarthy, L. et al. MEK5 overexpression is associated with metastatic prostate cancer, and stimulates proliferation, MMP-9 expression and invasion. Oncogene 22, 1381–1389 (2003). https://doi.org/10.1038/sj.onc.1206154
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206154
Keywords
This article is cited by
-
Etiopathogenic role of ERK5 signaling in sarcoma: prognostic and therapeutic implications
Experimental & Molecular Medicine (2023)
-
Aberrant MEK5 signalling promotes clear cell renal cell carcinoma development via mTOR activation
Journal of Cancer Research and Clinical Oncology (2022)
-
The ERK5/NF-κB signaling pathway targets endometrial cancer proliferation and survival
Cellular and Molecular Life Sciences (2022)
-
Reversal of ABCB1-related multidrug resistance by ERK5-IN-1
Journal of Experimental & Clinical Cancer Research (2020)
-
Targeting MEK5 impairs nonhomologous end-joining repair and sensitizes prostate cancer to DNA damaging agents
Oncogene (2020)