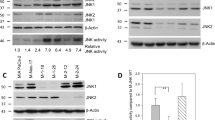
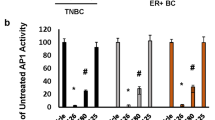
Abstract
Based on the findings that the overexpression of the wild-type Gα12 (Gα12WT) result in the oncogenic transformation of NIH3T3 cells in a serum-dependent manner, a model system has been established in which the mitogenic and subsequent cell transformation pathways activated by Gα12 can be turned on or off by the addition or removal of serum. Using this model system, our previous studies have shown that the stimulation of Gα12WT or the expression of an activated mutant of Gα12 (Gα12QL) leads to increased cell proliferation and subsequent oncogenic transformation of NIH3T3 cells, as well as persistent activation of Jun N-terminal kinases (JNKs). In the present studies, we show that the stimulation of Gα12WT or the expression of Gα12QL results in a potent inhibition of p38MAPK, and that the mechanism by which Gα12 inhibits p38MAPK activity involves the dual specificity kinases upstream of p38MAPK. The results indicate that Gα12 attenuates the activation of MKK3 and MKK4, which are known to stimulate only p38MAPK or p38MAPK and JNK, respectively. The results also suggest that Gα12 activates JNKs specifically through the stimulation of the JNK-specific upstream kinase MKK7. These findings demonstrate for the first time that Gα12 differentially regulates JNK and p38MAPK by specifically activating MKK7, while inhibiting MKK3 and MKK4 in NIH3T3 cells. Since the stimulation of p38MAPK is often associated with apoptotic responses, our findings suggest that Gα12 stimulates cell proliferation and neoplastic transformation of NIH3T3 cells by attenuating p38MAPK-associated apoptotic responses, while activating the mitogenic responses through the stimulation of ERK- and JNK-mediated signaling pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Abbreviations
- G protein:
-
guanine nucleotide-binding protein
- JNK:
-
Jun N-terminal kinase
- MAPK:
-
mitogen-activated protein kinase
- p38MAPK:
-
p38-MAPK
- MKK:
-
MAP- kinase
References
Avdi NJ, Malcolm KC, Nick JA and Worthen GS . (2002). J. Biol. Chem., 277, 40687–40696.
Chan AN-L, Fleming TP, McGovern ES, Chedid M, Miki T and Aaronson SA . (1993). Mol. Cell. Biol., 13, 762–768.
Collins LR, Minden A, Karin M and Brown JH . (1996). J. Biol. Chem., 271, 17349–17353.
Derijard B, Raingeaud J, Barrett T, Wu I-H, Han J, Ulevitch RJ and Davis RJ . (1995). Science, 267, 682–685.
Dermott JM and Dhanasekaran N . (2002). Meth. Enzymol., 344, 298–309.
Dhanasekaran N and Dermott JM . (1996). Cell. Signal., 8, 235–245.
Dhanasekaran N and Reddy EP . (1998). Oncogene, 17, 1447–1455.
Fleming Y, Armstrong CG, Morrice N, Peterson A, Goedert M and Cohen P . (2000). Biochem. J., 352, 145–154.
Heidenreich KA and Kummer JL . (1996). J. Biol. Chem., 271, 9891–9894.
Jiang H, Wu D and Simon MI . (1993). FEBS Lett., 3, 319–322.
Kummer JL, Rao PK and Heidenreich KA . (1997). J. Biol. Chem., 272, 20490–20494.
Lawler S, Fleming Y, Goedert M and Cohen P . (1998). Curr. Biol., 8, 1387–1390.
Lisnock J, Griffin P, Calaycay J, Frantz B, Parsons J, O'Keefe SJ and LoGrasso P . (2000). Biochemistry, 39, 3141–4148.
Meigs TE, Fields TA, Mc Kee DD and Casey PJ . (2001). Proc. Natl. Acad. Sci. USA, 98, 519–524.
Mitsui H, Takuwa N, Kurokawa K, Exton JH and Takuwa T . (1997). J. Biol. Chem., 272, 4904–4910.
Radhika V and Dhanasekaran N . (2001). Oncogene, 20, 1607–1614.
Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ and Davis RJ . (1995). J. Biol. Chem., 270, 7420–7426.
Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B and Davis RJ . (1996). Mol. Cell. Biol., 16, 1247–1255.
Vara Prasad MVVS, Dermott JM, Heasley LE, Johnson GL and Dhanasekaran N . (1995). J. Biol. Chem., 270, 18655–18659.
Vara Prasad MVVS, Shore SK and Dhanasekaran N . (1994). Oncogene, 9, 2425–2429.
Voyno-Yasenetskaya TA, Faur MP, Ahn NG and Bourne HR . (1996). J. Biol. Chem., 271, 21081–21087.
Voyno-Yasenetskaya T, Pace AM and Bourne HR . (1994). Oncogene, 9, 2559–2565.
Weston CR, Lambright DG and Davis RJ . (2002). Science, 296, 2345–2347.
Xu N, Bradley L, Ambudkar I and Gutkind JS . (1993). Proc. Natl. Acad. Sci. USA, 90, 11354–11358.
Yamaguchi Y, Katoh H, Mori K and Nagishi M . (2002). Curr. Biol., 12, 1353–1358.
Yang D, Tournier C, Wusk M, Lu HT, Xu J, Davis RJ and Flavell RA . (1997). Proc. Natl. Acad. Sci. USA, 94, 3004–3009.
Acknowledgements
This work was supported by a grant from NIH (GM49897) and a partial support to CHL by Hanyang University for the program year of 2002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dermott, J., Ha, J., Lee, C. et al. Differential regulation of Jun N-terminal kinase and p38MAP kinase by Gα12. Oncogene 23, 226–232 (2004). https://doi.org/10.1038/sj.onc.1207009
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207009
Keywords
This article is cited by
-
Gα12/13 signaling in metabolic diseases
Experimental & Molecular Medicine (2020)
-
G protein alpha 12
AfCS-Nature Molecule Pages (2010)
-
The G12 family proteins upregulate matrix metalloproteinase-2 via p53 leading to human breast cell invasion
Breast Cancer Research and Treatment (2010)
-
G Protein regulation of MAPK networks
Oncogene (2007)
-
Mitogenic signaling by lysophosphatidic acid (LPA) involves Gα12
Oncogene (2005)