Abstract
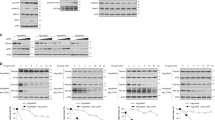
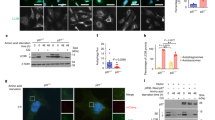
Ras proteins exert a pivotal regulatory function in signal transduction involved in cell proliferation and their activation mutation leads to malignant cell transformation. However, the role of Ras proteins in autophagy, an intracellular protein degradation process in cell growth control is unknown. In the present study, we demonstrate that the degradation of long-lived proteins in NIH3T3 cells in response to nutrient starvation was significantly suppressed by oncogenic RasVal12 transformation in a rapamycin (mTOR inhibitor)-sensitive manner. Morphologic observations also show the decrease in the formation of autophagic vacuoles upon the Ras transformation. Furthermore, epidermal growth factor or serum downregulated the protein degradation induced by serum starvation and the dominant-negative RasAsn17 mutant counteracted this suppressive effect, indicating that Ras mediates the growth factor downregulation of autophagy. The suppression of protein degradation by the activated RasVal12 was mediated by the class I phosphatidyl inositol 3-kinase (PI3-kinase), but not either or Raf Ral GDS. Consistent with this, RasVal12 and class I PI3-kinase inhibited the rate of autophagic sequestration of LDH. These data suggest that Ras plays a critical role as a negative regulator for nutrient deprivation-induced autophagy through the class I PI3-kinase signaling pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Arico S, Petitot A, Bauvy C, Dubbellhuis PF, Meijer AJ, Codogno P and Ogier-Denis E . (2001). J. Biol. Chem., 276, 35243–35246.
Ballard FJ, Wong SSC, Knowles SE, Partridge NC, Martin TJ, Wood CM and Gunn JM . (1980). J. Cell Physiol., 105, 335–346.
Blommaart EFC, Luiken JJFP and Meijer AJ . (1997). Histochem. J., 29, 365–385.
Dunn WA . (1990). J. Cell Biol., 110, 1923–1933.
Dunn WA . (1994). Trends Cell Biol., 4, 139–143.
Fleischman LF, Chawala SB and Cantley L . (1986). Science, 231, 407–410.
Franch HA, Sooparb S and Du J . (2001). J. Biol. Chem., 276, 19126–19131.
Furuta S, Miura K, Copeland T, Shang WH, Oshima A and Kamata T . (2002). Oncogene, 21, 7060–7066.
Houri JJ, Ogier-Denis E, De Stefanis D, Bauvy C, Baccino FM, Isidoro C and Codogno P . (1995). Biochem. J., 309, 521–527.
Kamata T and Kung HF . (1988). Proc. Natl. Acad. Sci. USA, 85, 5799–5803.
Khwaja A, Rodriguez-Viciana P, Wennström S, Warne PH and Downward J . (1997). EMBO J., 16, 2783–2793.
Kihara A, Kabeya Y, Ohsumi Y and Yoshimori T . (2001). EMBO Rep., 21, 330–335.
Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H and Levine B . (1999). Nature, 402, 672–676.
Ogier-Denis E and Codogno P . (2003). Biochim. Biophys. Acta, 1366, 177–196.
Ohkuma S and Poole B . (1978). Proc. Natl. Acad. Sci. USA, 75, 3327–3331.
Oldham S and Hafen E . (2003). Trends Cell Biol., 13, 79–85.
Pattingre S, Baury C and Codogno P . (2003). J. Biol. Chem., 278, 16667–16674.
Petiot A, Ogier-Denis E, Blommaart EFC, Meijer AJ and Codogno P . (2000). J. Biol. Chem., 275, 992–998.
Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D and Goldberg AL . (1994). Cell, 78, 761–771.
Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A and Downward J . (1997). Cell, 89, 457–467.
Schwarze PE and Seglen PO . (1985). Exp. Cell Res., 157, 15–28.
Seglen PO and Gordon PB . (1982). Proc. Natl. Acad. Sci. USA, 79, 1889–1892.
Sluijters DA, Dubbelhuis PF, Blommaart EF and Meijer AJ . (2000). Biochem. J., 351, 545–550.
Yokota S, Himeno M, Roth J, Brada D and Kato K . (1993). Eur. J. Cell Biol., 62, 372–383.
Acknowledgements
We thank Drs Y Fukui and J Downward for generous gifts of plasmid DNAs. We also thank Mrs F Ushiyama for preparation of the manuscript. This research was partially supported by Grant in Aid from Japanese Ministry of Science and Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Furuta, S., Hidaka, E., Ogata, A. et al. Ras is involved in the negative control of autophagy through the class I PI3-kinase. Oncogene 23, 3898–3904 (2004). https://doi.org/10.1038/sj.onc.1207539
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207539
Keywords
This article is cited by
-
SQSTM1/p62 loss reverses the inhibitory effect of sunitinib on autophagy independent of AMPK signaling
Scientific Reports (2019)
-
Autophagy suppresses Ras-driven epithelial tumourigenesis by limiting the accumulation of reactive oxygen species
Oncogene (2017)
-
BRAF V600E-dependent role of autophagy in uveal melanoma
Journal of Cancer Research and Clinical Oncology (2017)
-
Autophagy induction by SIRT6 is involved in oxidative stress-induced neuronal damage
Protein & Cell (2016)
-
HTLV-1 Tax deregulates autophagy by recruiting autophagic molecules into lipid raft microdomains
Oncogene (2015)