Abstract
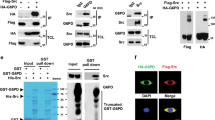
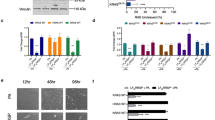
Previously, we showed that Src tyrosine kinases are activated early in the development of human colon cancer and are suppressed as intestinal cells differentiate. We identified RACK1 as an endogenous substrate, binding partner and inhibitor of Src. Here we show (by overexpressing RACK1, depleting Src or RACK1 and utilizing cell-permeable peptides that perturb RACK1's interaction with Src) that RACK1 regulates growth of colon cells by suppressing Src activity at G1 and mitotic checkpoints, and consequently delaying cell cycle progression. Activated Src rescues RACK1-inhibited growth of HT-29 cells. Conversely, inhibiting Src abolishes growth promoted by RACK1 depletion in normal cells. Two potential mechanisms whereby RACK1 regulates mitotic exit are identified: suppression of Src-mediated Sam68 phosphorylation and maintenance of the cyclin-dependent kinase (CDK) 1-cyclin B complex in an active state. Our results reveal novel mechanisms of cell cycle control in G1 and mitosis of colon cells. The significance of this work lies in the discovery of a mechanism by which the growth of colon cancer cells can be slowed, by RACK1 suppression of an oncogenic kinase at critical cell cycle checkpoints. Small molecules that mimic RACK1 function may provide a powerful new approach to the treatment of colon cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L et al. (2000). SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol 20: 9018–9027.
Bromann PA, Korkaya H, Courtneidge SA . (2004). The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 23: 7957–7968.
Cartwright C . (1998). Intestinal cell growth control: role of Src tyrosine kinases. Gastroenterology 114: 1335–1338.
Cartwright CA, Eckhart W, Simon S, Kaplan PL . (1987). Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell 49: 83–91.
Cartwright CA, Kamps MP, Meisler AI, Pipas JM, Eckhart W . (1989). pp60c-src activation in human colon carcinoma. J Clin Invest 83: 2025–2033.
Cartwright CA, Meisler AI, Eckhart W . (1990). Activation of the pp60c-src protein kinase is an early event in colonic carcinogenesis. Proc Natl Acad Sci USA 87: 558–562.
Chang BY, Chiang M, Cartwright CA . (2001). The interaction of Src and RACK1 is enhanced by activation of protein kinase C and tyrosine phosphorylation of RACK1. J Biol Chem 276: 20346–20356.
Chang BY, Conroy KB, Machleder EM, Cartwright CA . (1998). RACK1, a receptor for activated C kinase and a homolog of the beta subunit of G proteins, inhibits activity of src tyrosine kinases and growth of NIH 3T3 cells. Mol Cell Biol 18: 3245–3256.
Chang BY, Harte RA, Cartwright CA . (2002). RACK1: a novel substrate for the Src protein-tyrosine kinase. Oncogene 21: 7619–7629.
Derry JJ, Richard S, Valderrama Carvajal H, Ye X, Vasioukhin V, Cochrane AW et al. (2000). Sik (BRK) phosphorylates Sam68 in the nucleus and negatively regulates its RNA binding ability. Mol Cell Biol 20: 6114–6126.
Di Fruscio M, Chen T, Richard S . (1999). Characterization of Sam68-like mammalian proteins SLM-1 and SLM-2: SLM-1 is a Src substrate during mitosis. Proc Natl Acad Sci USA 96: 2710–2715.
Dorn GW, Mochly-Rosen D . (2002). Intracellular transport mechanisms of signal transducers. Annu Rev Physiol 64: 407–429.
Fleury S, Rizzardi GP, Chapuis A, Tambussi G, Knabenhans C, Simeoni E et al. (2000). Long-term kinetics of T cell production in HIV-infected subjects treated with highly active antiretroviral therapy. Proc Natl Acad Sci USA 97: 5393–5398.
Haegebarth A, Heap D, Bie W, Derry JJ, Richard S, Tyner AL . (2004). The nuclear tyrosine kinase BRK/Sik phosphorylates and inhibits the RNA-binding activities of the Sam68-like mammalian proteins SLM-1 and SLM-2. J Biol Chem 279: 54398–54404.
Itoh M, Haga I, Li QH, Fujisawa J . (2002). Identification of cellular mRNA targets for RNA-binding protein Sam68. Nucleic Acids Res 30: 5452–5464.
Liu K, Li L, Nisson PE, Gruber C, Jessee J, Cohen SN . (2000). Neoplastic transformation and tumorigenesis associated with sam68 protein deficiency in cultured murine fibroblasts. J Biol Chem 275: 40195–40201.
Lukong KE, Larocque D, Tyner AL, Richard S . (2005). Tyrosine phosphorylation of sam68 by breast tumor kinase regulates intranuclear localization and cell cycle progression. J Biol Chem 280: 38639–38647.
Lukong KE, Richard S . (2003). Sam68, the KH domain-containing superSTAR. Biochim Biophys Acta 1653: 73–86.
Mamidipudi V, Chang BY, Harte RA, Lee KC, Cartwright CA . (2004a). RACK1 inhibits the serum- and anchorage-independent growth of v-Src transformed cells. FEBS Lett 567: 321–326.
Mamidipudi V, Zhang J, Lee KC, Cartwright CA . (2004b). RACK1 regulates G1/S progression by suppressing Src kinase activity. Mol Cell Biol 24: 6788–6798.
Miller LD, Lee KC, Mochly-Rosen D, Cartwright CA . (2004). RACK1 regulates Src-mediated Sam68 and p190RhoGAP signaling. Oncogene 23: 5682–5686.
Moasser MM, Srethapakdi M, Sachar KS, Kraker AJ, Rosen N . (1999). Inhibition of Src kinases by a selective tyrosine kinase inhibitor causes mitotic arrest. Cancer Res 59: 6145–6152.
Park J, Cartwright CA . (1995). Src activity increases and Yes activity decreases during mitosis of human colon carcinoma cells. Mol Cell Biol 15: 2374–2382.
Paul CP, Good PD, Winer I, Engelke DR . (2002). Effective expression of small interfering RNA in human cells. Nat Biotechnol 20: 505–508.
Pillay I, Nakano H, Sharma SV . (1996). Radicicol inhibits tyrosine phosphorylation of the mitotic Src substrate Sam68 and retards subsequent exit from mitosis of Src-transformed cells. Cell Growth Differ 7: 1487–1499.
Porter LA, Donoghue DJ . (2003). Cyclin B1 and CDK1: nuclear localization and upstream regulators. Prog Cell Cycle Res 5: 335–347.
Riley D, Carragher NO, Frame MC, Wyke JA . (2001). The mechanism of cell cycle regulation by v-Src. Oncogene 20: 5941–5950.
Ron D, Luo J, Mochly-Rosen D . (1995). C2 region-derived peptides inhibit translocation and function of beta protein kinase C in vivo. J Biol Chem 270: 24180–24187.
Ron D, Mochly-Rosen D . (1995). An autoregulatory region in protein kinase C: the pseudoanchoring site. Proc Natl Acad Sci USA 92: 492–496.
Stebbins EG, Mochly-Rosen D . (2001). Binding specificity for RACK1 resides in the V5 region of beta II protein kinase C. J Biol Chem 276: 29644–29650.
Taylor SJ, Resnick RJ, Shalloway D . (2004). Sam68 exerts separable effects on cell cycle progression and apoptosis. BMC Cell Biol 5: 5.
Thomas SM, Brugge JS . (1997). Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13: 513–609.
Wang LL, Richard S, Shaw AS . (1995). P62 association with RNA is regulated by tyrosine phosphorylation. J Biol Chem 270: 2010–2013.
Acknowledgements
We thank Jenny Cheng for many helpful discussions, assistance with data analysis and critical review of the manuscript. This work was supported by NIH Grant DK43743 (CAC) and NIH Digestive Disease Center Grant DK56339.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Mamidipudi, V., Dhillon, N., Parman, T. et al. RACK1 inhibits colonic cell growth by regulating Src activity at cell cycle checkpoints. Oncogene 26, 2914–2924 (2007). https://doi.org/10.1038/sj.onc.1210091
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210091
Keywords
This article is cited by
-
RACK1 facilitates breast cancer progression by competitively inhibiting the binding of β-catenin to PSMD2 and enhancing the stability of β-catenin
Cell Death & Disease (2023)
-
Skin chronological aging drives age-related bone loss via secretion of cystatin-A
Nature Aging (2022)
-
Dual functions of Rack1 in regulating Hedgehog pathway
Cell Death & Differentiation (2020)
-
RACK1 interaction with c-Src is essential for osteoclast function
Experimental & Molecular Medicine (2019)
-
RACK1 promotes tumorigenicity of colon cancer by inducing cell autophagy
Cell Death & Disease (2018)