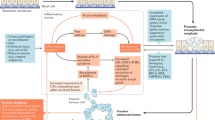
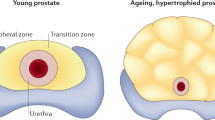
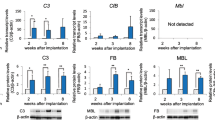
Abstract
Prostasomes are submicron secretory granules synthesized, stored and secreted by the epithelial cells of the human prostate gland. They are membrane-surrounded also in their extracellular appearance and the membrane architecture is composite. They are believed to be life-giving and act as protectors of the spermatozoa in the lower and upper female genital tract on their way to the ovum. Hence, the prostasomes are immunosuppressive and inhibitory of complement activation. Further, they promote sperm's forward motility and have antioxidant and antibacterial capacities. The prostasomes with their many composite abilities seem to turn against the host cell after the age of 50 y being conducive to the transition of the normal prostate epithelial cell into a neoplastic cell and therewith lay the foundations of the very high prevalence of prostate cancer of men of more than 50 y of age.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hsing AW, Tsao L, Devesa SS . International trends and patterns of prostate cancer incidence and mortality. Int J Cancer 2000; 85: 60–67.
Yatani R et al. Geographic pathology of latent prostatic carcinoma. Int J Cancer 1982; 29: 611–616.
Angwafo FF . Migration and prostate cancer: an international perspective. J Natl Med Assoc 1998; 90: 720–723.
Watanabe M et al. Comparative studies of prostate cancer in Japan versus the United States. A review. Rev Urol Oncol 2000; 5: 274–283.
Takahashi H et al. Prevalence of androgen receptor gene mutations in latent prostatic carcinomas from Japanese men. Cancer Res 1995; 55: 1621–1624.
Miller GJ, Torkko KC . Natural history of prostate cancer—epidemiologic considerations. Epidemiol Rev 2001; 23: 14–18.
Ormsby AH, Haskell R, Jones D, Goldblum JR . Primary seminal vesicle carcinoma: an immunohistochemical analysis of four cases. Mod Pathol 2000; 13: 46–51.
Ronquist G, Brody I . The prostasome: its secretion and function in man. Biochim Biophsys Acta 1985; 822: 203–218.
Ronquist G, Brody I, Gottfries A, Stegmayr B . An Mg2+ and Ca2+ -stimulated adenosine triphosphatase in human prostatic fluid Part I and Part II. Andrologia 1978; 10: 261–272. and 427–431.
Nilsson BO et al. Monoclonal antibodies against human prostasomes. Prostate 1998; 35: 178–184.
Ronquist G, Hedström M . Restoration of detergent-inactivated adenosine triphosphatase activity of human prostatic fluid with concanavalin A. Biochim Biophys Acta 1977; 483: 483–486.
Ronquist G . Effect of modulators on prostasome membrane-bound ATPase in human seminal plasma. Eur J Clin Invest 1987; 17: 231–236.
Stegmayr B, Gottfries A, Ronquist G, Brody I . Reduced activity of Mg2+ -and Ca2+ dependent adenosine triphosphatase in seminal fluid of patients with oligozoospermia. Scand J Urol Nephrol 1980; 14: 129–134.
Brody I, Ronquist G, Gottfries A, Stegmayr B . Abnormal deficiency of both Mg2+ and Ca2+ -dependent adenosine triphosphatase and secretory granules and vesicles in human seminal plasma. Scand J Urol Nephrol 1981; 15: 85–90.
Stegmayr B, Berggren PO, Ronquist G, Hellman B . Calcium, magnesium and zinc contents in organelles of prostatic origin in human seminal plasma. Scand J Urol Nephrol 1982; 16: 199–203.
Wilson MJ et al. Prostate specific origin of dipeptidylpeptidase IV (CD 26) in human seminal plasma. J Urol 1998; 160: 1905–1909.
Schrimpf SP et al. Identification of dipeptidylpeptidase IV as the antigen of a monoclonal anti-prostasome antibody. Prostate 1999; 38: 35–39.
Renneberg H et al. Immunohistochemistry of prostasomes from human semen. Prostate 1997; 30: 98–106.
Sahlén GE et al. Ultrastructure of the secretion of prostasomes from benign and malignant epithelial cells in tghe prostate. Prostate 2002; 53: 192–199.
Carlsson L et al. Characteristics of human prostasomes isolated from three different sources. Prostate 2003; 54: 322–330.
Weisser H, Krieg M . Lipid composition in epithelium and stroma of human benign prostatic hyperplasia. Prostate 1997; 30: 41–46.
Mack SR, Everingham J, Zaneveld LJ . Isolation and partial characterization of the plasma membrane from human spermatozoa. J Exp Zool 1986; 240: 127–136.
Poulos A, White IG . The phospholipid composition of human spermatozoa and seminal plasma. J Reprod Fertil 1973; 35: 265–272.
Seitz J, Keppler C, Rausch U, Aumuller G . Immunohistochemistry of secretory transglutaminase from rodent prostate. Histochemistry 1990; 93: 525–530.
Steinhoff M et al. Hormonally induced changes in apocrine secretion of transglutaminase in the rat dorsal prostate and coagulating gland. Eur J Cell Biol 1994; 65: 49–59.
Utleg AG et al. Proteomic analysis of human prostasomes. Prostate 2003; 56: 150–161.
Ronquist G, Jin M, Schrimpf S, Nilsson BO . Prostasomes and human reproduction. In: Motta PM (ed) Microscopy of reproduction and development: A dynamic approach. Antonio Delfino Editore: Rome 1997, pp 213–219.
Ronquist G, Nilsson BO . Prostasomes. Wenner-Gren International Series, Vol 81. Portland Press Ltd: London, 2002, 157pp.
Ronquist G, Frithz G . Prostasomes in human semen contain ADP and GDP. Acta Eur Fertil 1986; 17: 273–276.
Debnath D, Bhattacharya S, Chakrabarti A . Phospholipid assisted folding of a denatured heme protein: effect of phosphatidylethanolamine. Biochem Biophys Res Commun 2003; 301: 979–984.
Arvidson G, Ronquist G, Wikander G, Öjteg AC . Human prostasome membranes exhibit very high cholesterol/phospholipid ratios yielding high molecular ordering. Biochim Biophys Acta 1989; 984: 167–173.
Arienti G et al. Fatty acid pattern of human prostasome lipid. Arch Biochem Biophys 1998; 358: 391–395.
Fabiani R, Ronquist G . Association of some hydrolytic enzymes with the prostasome membrane and their differential responses to detergent and PIPLC treatment. Prostate 1995; 27: 95–101.
Ronquist G . Zinc ion stimulation of ATP cleavage by prostasomes from human seminal plasma. Urol Int 1988; 43: 334–340.
Ronquist G, Frithz G . Inhibition of Zn2+ -dependent ATPase in prostasome membrane by nonsaturated, long-chain fatty acids. Urol Int 1992; 48: 184–186.
Majumbder GC . Occurrence of a cyclic AMP-dependent protein kinase on the outer surface of rat epididymal spermatozoa. Biochem Biophys Res Commun 1978; 83: 829–836.
Stegmayr B, Brody I, Ronquist G . A biochemical and ultrastructural study on the endogenous protein kinase activity of secretory granule membranes of prostatic origin in human seminal plasma. J Ultrastruct Res 1982; 78: 206–214.
Wilson MJ, Steer RC, Kaye KW . Presence and characterization of two protein kinase activities in human seminal fluid. Biochim Biophys Acta 1982; 700: 206–212.
Wilson MJ, Kaye KW . Tissue sources of protein kinase activities in human seminal fluid: studies of normal, oligozoospermic and vasectomized men. Fertil Steril 1983; 40: 105–109.
Wilson MJ, Steer RC, Kaye KW . Protein kinase activities in human prostatic secretion: biochemical characterization and effect of prostatitis. J Lab Clin Med 1984; 103: 905–911.
Lilja H, Weber H . Glutamyltransferase bound to prostatic subcellular organelles and in free form in human seminal plasma. Scand J Clin Lab Invest 1983; 43: 307–312.
Ronquist G, Frithz G, Jansson Å . Prostasome membrane associated enzyme activities and semen parameters in men attending an infertility clinic. Urol Int 1988; 43: 133–138.
Tvedt KE, Kopstad G, Haugen OA, Halgunset J . Subcellular concentrations of calcium, zinc and magnesium in benign nodular hyperplasia of the human prostate: x-ray micro-analysis of freeze-dried cryosections. Cancer Res 1987; 47: 323–328.
Vivacqua A et alProstasomes as zinc ligands in human seminal plasma. Int J Androl 2003, in press.
Ronquist G, Nilsson BO, Hjertén S . Interaction between prostasomes and spermatozoa from human semen. Arch Androl 1990; 24: 147–157.
Carlini E, Palmerini CA, Cosmi EV, Arienti G . Fusion of sperm with prostasomes: effects on membrane fluidity. Arch Biochem Biophys 1997; 343: 6–12.
Minelli A et al. CD 26 and adenosine deaminase interaction: its role in the fusion between horse membrane vesicles and spermatozoa. Biol Reprod 1999; 61: 802–808.
Wang J et al. Prostasome-like granules from the PC-3 prostate cancer cell line increase the motility of washed human spermatozoa and adhere to the sperm. Eur J Obstet Gynecol Reprod Biol 2001; 96: 88–97.
Arienti G, Carlini E, Palmerini CA . Fusion of human sperm to prostasomes at acidic pH. J Membr Biol 1997; 155: 89–94.
Arienti G et al. Prostasome to sperm transfer of CD 13/aminopeptidase N (EC 3.411.2). Biochim Biophys Acta 1997; 1336: 533–538.
Palmerini CA, Carlini E, Nicolucci A, Arienti G . Increase of human spermatozoa intracellular Ca2+ concentration after fusion with prostasomes. Cell Calcium 1999; 25: 291–296.
Fabiani R et al. Promotive effect by prostasomes on normal human spermatozoa exhibiting no forward motility due to buffer washings. Eur J Obstet Gynecol Reprod Biol 1994; 57: 181–188.
Fabiani R, Johansson L, Lundkvist Ö, Ronquist G . Prolongation and improvement of prostasome promotive effect on sperm forward motility. Eur J Obstet Gynecol Reprod Biol 1995; 58: 191–198.
Fabiani R, Johansson L, Lundkvist Ö, Ronquist G . Enhanced recruitment of motile spermatozoa by prostasome inclusion in swim-up medium. Hum Reprod 1994; 9: 1485–1489.
Carlsson L, Ronquist G, Stridsberg M, Johansson L . Motility stimulant effects of prostasome inclusion in swim-up medium on cryopreserved human spermatozoa. Arch Androl 1997; 38: 215–221.
Skibinski G, Kelly RW, Harkiss D, James K . Immunosuppression by human seminal plasma—extracellular organelles (prostasomes) modulate activity of phagocytic cells. Am J Reprod Immunol 1992; 28: 97–103.
Kelly RW . Immunosuppressive mechanisms in semen: implications for contraception. Hum Reprod 1995; 10: 1686–1693.
Kelly RW et al. Extracellular organelles (prostasomes) are immunosuppressive components of human semen. Clin Exp Immunol 1991; 86: 550–556.
Rooney IA et al. Physiologic relevance of the membrane attack complex inhibitory protein CD 59 in human seminal plasma: CD 59 is present on extracellular organelles (prostasomes), binds cell membranes and inhibits complement-mediated lysis. J Exp Med 1993; 177: 1409–1420.
Iwasaki A, Gagnon C . Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril 1992; 57: 409–416.
Alvarez JG, Storey BT . Role of glutathione peroxidase in protecting mammalian spermatozoa from loss of motility caused by spontaneous lipid peroxidation. Gamete Res 1989; 23: 77–90.
Saez F, Motta C, Boucher D, Grizard G . Antioxidant capacity of prostasomes in human semen. Mol Hum Reprod 1998; 4: 667–672.
Saez F, Motta C, Boucher D, Grizard G . Prostasomes inhibit the NADPH oxidase activity of human neutrophils. Mol Hum Reprod 2000; 6: 883–891.
Carlsson L et al. Antibacterial activity of human prostasomes. Prostate 2000; 44: 279–286.
Boon T, van der Bruggen P . Human tumour antigens recognized by T lymphocytes. J Exp Med 1996; 183: 725–729.
Rosenberg SA . A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity 1999; 10: 281–287.
Gilboa E . The makings of a tumour rejection antigen. Immunity 1999; 11: 263–270.
Yamakawa M et al. Protection of thyroid cancer cells by complement-regulatory factors. Cancer 1994; 73: 2808–2817.
Pangburn MK . Activation of complement via the alternative pathway. Fed Proc 1983; 42: 139–143.
Hakulinen J, Meri S . Expression and function of the complement membrane attack complex inhibitor protectin (CD 59) on human breast cancer cells. Lab Invest 1994; 71: 820–827.
Jarvis GA et al. Expression and function of the complement membrane attack complex inhibitor protectin (CD 59) in human prostate cancer. Int J Cancer 1997; 71: 1049–1055.
Jurianz K et al. Complement resistance of tumour cells: basal and induced mechanisms. Mol Immunol 1999; 36: 929–939.
Donin N et al. Complement resistance of human carcinoma cells depends on membrane regulatory proteins, protein kinases and sialic acid. Clin Exp Immunol 2003; 131: 254–263.
Babiker AA, Ronquist G, Nilsson UR, Nilsson B . Transfer of prostasomal CD 59 to CD 59-deficient red blood cells results in protection against complement-mediated hemolysis. Am J Reprod Immunol 2002; 47: 183–192.
Polak JM, Bloom SR . The diffuse neuroendocrine system. Studies of this newly discovered controlling system in health and disease. J Histochem Cytochem 1979; 27: 1398–1400.
Aumuller G et al. Neurogenic origin of human prostate endocrine cells. Urology 1999; 53: 1041–1048.
Stridsberg M, Fabiani R, Lukinius A, Ronquist G . Prostasomes are neuroendocrine-like vesicles in human semen. Prostate 1996; 29: 287–295.
Kaleczyc J et al. Immunohistochemical properties of nerve fibres supplying accessory male genital glands in the pig. A colocalisation study. Histochem Cell Biol 1999; 111: 217–228.
Guijarro LG et al. Modulation of cyclic AMP and inositol phosphate production in rat prostatic cultures by VIP/PACAP, ATP and carbachole: role in prostatic proliferation. Ann N Y Acad Sci 1996; 805: 723–728.
Gkonos PJ, Guo F, Burnstein KL . Type 1 vasoactive intestinal peptide receptor expression in PC3/AR cells is evidence of prostate epithelial differentiation. Prostate 2000; 42: 137–144.
Reubi JC et al. Vasoactive intestinal peptide/pituitary adenylate cyclase–activating peptide receptor subtypes in human tumours and their tissue origin. Cancer Res 2000; 60: 3105–3112.
Adrian TE et al. Neuropeptide Y in the human male genital tract. Life Sci 1984; 35: 2643–2648.
Dixon JS, Jen PY, Gosling JA . The distribution of vesicular acetylcholine transporter in the human male genitourinary organs and its colocalisation with neuropeptide Y and nitric oxide synthase. Neurourol Urodyn 2000; 19: 185–194.
DeClerck YA, Laug WE . Cooperation between matrix metalloproteinases and the plasminogen activator-plasmin system in tumour progression. Enzyme Protein 1996; 49: 72–84.
de Vries TJ, van Muijen GN, Ruiter DJ . The plasminogen activation system in tumour invasion and metastasis. Pathol Res Pract 1996; 192: 718–733.
Gonzales-Gronow M et al. Interaction of plasminogen with dipeptidyl peptidase IV initiates a signal transduction mechanism which regulates expression of matrix proteinase-9 by prostate cancer cells. Biochem J 2001; 355: 397–407.
Kenny AJ et al. Dipeptidyl peptidase IV, a kidney brush-border serine peptidase. Biochem J 1976; 157: 169–182.
Walter R, Simmons WH, Yoshimoto T . Proline specific endo- and exopeptidases. Mol Cell Biochem 1980; 30: 111–127.
Heins J et al. Mechanism of proline-specific proteinases: (I) substrate specificity of dipeptidyl peptidase IV from pig kidney and proline-specific endopeptidase from Flavobacterium meningosepticum. Biochem Biophys Acta 1988; 954: 161–169.
Puschel G, Mentlein R, Heymann E . Isolation and characterization of dipeptidyl peptidase IV from human placenta. Eur J Biochem 1982; 126: 359–365.
Ogata S, Misumi Y, Ikehara Y . Primary structure of rat liver dipeptidyl peptidase IV deduced from its cDNA and identification of the NH2-terminal signal sequence as the membrane-anchoring domain. J Biol Chem 1989; 264: 3596–3601.
Vanhoof G et al. Distribution of proline-specific aminopeptidases in human tissues and body fluids. Eur J Clin Chem Clin Biochem 1992; 30: 333–338.
Heymann E, Mentlein R . Liver dipeptidyl aminopeptidase IV hydrolyzes substance P. FEBS Lett 1978; 91: 360–364.
Nagakawa O et al. Effect of prostatic neuropeptides on invasion and migration of PC-3 prostate cancer cells. Cancer Lett 1998; 133: 27–33.
Cussenot O et al. Plasma neuroendocrine markers in patients with benign prostatic hyperplasia and prostatic carcinoma. J Urol 1996; 155: 1340–1343.
Vanhoof G et al. Proline motifs in peptides and their biological processing. FASEB J 1995; 9: 736–744.
Wilson MJ et al. Dipeptidyl peptidase IV activities are elevated in prostate cancers and adjacent benign hyperplastic glands. J Androl 2000; 21: 220–226.
Nemerson Y . Tissue factor and hemostasis. Blood 1988; 71: 1–8.
Fernández JA, Heeb MJ, Radtke KP, Griffin JH . Potent blood coagulant activity of human semen due to prostasome-bound tissue factor. Biol Reprod 1997; 56: 757–763.
Camerer E, Kolsto AB, Prydz H . Cell biology of tissue factor, the principal initiator of blood coagulation. Thromb Res 1996; 81: 1–41.
Francis JL, Biggerstaff J, Amirkhosravi A . Hemostasis and malignancy. Semin Thromb Hemost 1998; 24: 93–109.
Zhang Y et al. Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumour cells in mice. J Clin Invest 1994; 94: 1320–1327.
Sato Y et al. Tissue factor induces migration of cultured aortic smooth muscle cells. Thromb Haemost 1996; 75: 389–392.
Bromberg ME et al. Tissue factor promotes melanoma metastasis by a pathway independent of blood coagulation. Proc Natl Acad Sci USA 1995; 92: 8205–8209.
Gonzales-Gronow M, Gawdi G, Pizzo SV . Tissue factor is the receptor for plasminogen type 1 on 1-LN human prostate cancer cells. Blood 2002; 99: 4562–4567.
Andreasen PA, Kjoller L, Christensen L, Dutty MJ . The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 1997; 72: 1–22.
Ollivier V et al. Vascular endothelial growth factor production by fibroblasts in response to factor VIIa binding to tissue factor involves thrombin and factor Xa. Arterioscler Thromb Vasc Biol 2000; 20: 1374–1381.
Abdulkadir SA et al. Tissue factor expression and angiogenesis in human prostate carcinoma. Hum Pathol 2000; 31: 403–405.
Nilsson BO et al. Distribution of prostasomes in neoplastic epithelial prostate cells. Prostate 1999; 39: 36–40.
Soffer RL . Angiotensin-converting enzyme and the regulation of vasoactive peptides. Annu Rev Biochem 1976; 45: 73–94.
Krassnigg F et al. Investigations on the functional role of angiotensin converting enzyme (ACE) in human seminal plasma. Adv Exp Med Biol 1986; 198(Part A): 477–485.
O'Mahony OA et al. Angiotensin II in human seminal fluid. Hum Reprod 2000; 15: 1345–1349.
Fernandez LA, Twickler J, Mead A . Neovascularization produced by angiotensin II. J Lab Clin Med 1985; 105: 141–145.
Le Noble FA et al. The role of angiotensin II and prostaglandins in arcade formation in a developing microvascular network. J Vasc Res 1996; 33: 480–488.
Folkman J, Watson K, Ingber D, Hanahan D . Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 1989; 339: 58–61.
Paquet JL, Baudouin-Legros M, Brunelle G, Meyer P. Angiotensin II-induces proliferation of aortic myocytes in spontaneously hypertensive rats. J Hypertens 1990; 8: 565–572.
Daemen MJ, Lombardi DM, Bosman FT, Schwartz SM . Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res 1991; 68: 450–456.
Lyall F et al. Angiotensin II increases proto-oncogene expression and phosphoinositide turnover in vascular smooth muscle cells via the angiotensin II AT 1 receptor. J Hypertens 1992; 10: 1463–1469.
Sadishima J, Izumo S . Signal transduction pathways of angiotensin II-induced c-f gene expression in cardiac myocytes in vitro. Roles of phospholipid-derived second messengers. Circ Res 1993; 73: 424–438.
Zucker S, Cao J . Imaging metalloprotease activity in vivo. Nat med 2001; 7: 655–656.
Nagase H, Woessner Jr JF . Matrix metalloproteinases. J Biol Chem 1999; 274: 21491–21494.
Folkman J . The role of angiogenesis in tumour growth. Semin Cancer Biol 1992; 3: 65–71.
Menrad A, Speicher D, Wacker J, Herlyn M . Biochemical and functional characterization of aminopeptidase N expressed by human melanoma cells. Cancer Res 1993; 53: 1450–1455.
Saiki I et al. Role of aminopeptidase N (CD 13) in tumour-cell invasion and extracellular matrix degradation. Int J Cancer 1993; 54: 137–143.
Stearns ME, Stearns M . Immunohistochemical studies of activated matrix metalloproteinase-2 (MMP-2a) expression in human prostate cancer. Oncol Res 1996; 8: 63–67.
Kerkela E, Saarialho-Kere U . Matrix metalloproteinase in tumour progression: focus on basal and squamous cell skin cancer. Exp Dermatol 2003; 12: 109–125.
Gronberg H . Prostate cancer epidemiology. Lancet 2003; 361: 859–864.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ronquist, G., Nilsson, B. The Janus-faced nature of prostasomes: their pluripotency favours the normal reproductive process and malignant prostate growth. Prostate Cancer Prostatic Dis 7, 21–31 (2004). https://doi.org/10.1038/sj.pcan.4500684
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.pcan.4500684
Keywords
This article is cited by
-
Microfluidics facilitating the use of small extracellular vesicles in innovative approaches to male infertility
Nature Reviews Urology (2023)
-
Unlocking the mystery associated with infertility and prostate cancer: an update
Medical Oncology (2023)
-
Extracellular vesicles as a source of prostate cancer biomarkers in liquid biopsies: a decade of research
British Journal of Cancer (2022)
-
Communication between cells: exosomes as a delivery system in prostate cancer
Cell Communication and Signaling (2021)
-
An analysis of benign human prostate offers insights into the mechanism of apocrine secretion and the origin of prostasomes
Scientific Reports (2019)