Abstract
Oxymatrine is a traditional Chinese herbal product that exhibits anti-inflammatory effects in models of heart, brain and liver injury. We investigated the impact of oxymatrine in an acute model of intestinal injury and inflammation. Oxymatrine significantly decreased LPS-induced NF-κB-driven luciferase activity, correlating with diminished induction of Cxcl2, Tnfα and Il6 mRNA expression in rat IEC-6 and murine BMDC. Although oxymatrine decreased LPS-induced p65 nuclear translocation and binding to the Cxcl2 gene promoter, this effect was independent of IκBα degradation/phosphorylation. DSS-induced weight loss and histological damage were ameliorated in oxymatrine-treated C57BL/6-WT-mice. While this effect correlated with reduced colonic Il6 and Il1β mRNA accumulation, global NF-κB activity as measured in NF-κBEGFP mice was unaffected. Our data demonstrate that oxymatrine reduces LPS-induced NF-κB nuclear translocation and activity independently of IκBα status, prevents intestinal inflammation through blockade of inflammatory signaling and ameliorates overall intestinal inflammation in vivo.
Similar content being viewed by others
Introduction
Herbal products and traditional medicines are estimated to be the primary means of healthcare for up to 80% of the population in some Asian and African countries, with herbal treatments being the most popular1. Popularity for use of herbal products as complementary medication in western countries has risen dramatically in recent years and in the United States the use of herbal products is estimated at between 20-38% among adults2,3,4 while in some European countries this estimate rises to a range of 50–70%5. However, the lack of tight regulation of herbal products (production, purity and quality), unsustainable health claims and the lack of defined mechanisms of action prevents the incorporation of many herbal treatments into mainstream medicine2.
Oxymatrine, classified as a quinolizidine alkaloid, is an herbal product derived from the root of the Sophora flavescens Ait. plant6. Oxymatrine has been primarily used in China for the treatment of various human illnesses such as hepatitis B infections and liver fibrosis7,8. It has also been reported to provide a protective effect in various animal models of ischemia and reperfusion (I/R)-induced liver, heart and intestinal injury9,10,11. The molecular pathways by which oxymatrine mediates its protective effects remain unclear but appear to involve both inhibition of p38MAPK phosphorylation11,12 and decreased NF-κB subunit p65 protein expression13,14. In addition to the aforementioned protection from I/R-induced injury, it was reported that oxymatrine administration decreased dextran sulfate sodium (DSS) induced colitis in rats15, although the mechanism of action was not fully investigated.
Here we show that oxymatrine prevents LPS induced pro-inflammatory gene expression in bone marrow-derived dendritic cells (BMDC) and in intestinal epithelial cells (IEC). We demonstrate that oxymatrine dampens NF-κB activity by inhibiting LPS-induced nuclear translocation of p65, an effect independent of signal induced IκB phosphorylation/degradation. Importantly, oxymatrine ameliorates DSS-induced weight loss, histopathological scores and expression of Il1β and Il6 pro-inflammatory gene expression without global inhibition of NF-κB transcriptional activity.
Results
Oxymatrine inhibits LPS-induced pro-inflammatory cytokine expression in IECs and BMDCs
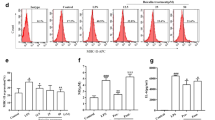
To investigate the effects of oxymatrine on pro-inflammatory cytokine production, we stimulated the rat intestinal epithelial cell (IEC)-6 line with LPS and treated cells using various concentrations of oxymatrine and measured expression of pro-inflammatory mediators. LPS-induced Cxcl2, Tnfα and Il6 mRNA expression were significantly reduced in presence of oxymatrine (p < 0.05) at 1 hr but only Cxcl2 remained inhibited by 4 hr ( Fig. 1 ). LPS-induced //1β mRNA accumulation was unaffected by oxymatrine treatment ( Fig. 1 ). To ensure that cell death was not responsible for the decreased LPS-induced cytokine expression by oxymatrine, we performed a cell viability assay. No significant difference in cell mortality was noticed in cells treated with oxymatrine or co-treated with oxymatrine and LPS compared to control cells (< 5% at 4 mg/ml) (data not shown).
Oxymatrine inhibits LPS-mediated Cxcl2, Il6 and Tnfα expression in IEC-6 Cells.
IEC-6 cells were stimulated for 1 and 4 hr with 5 μg/mL LPS ± 4 mg/mL OMT and real-time PCR performed to analyze Cxcl2, Tnfα, Il6 and Il1b mRNA accumulation standardized to Gapdh expression. Measurements expressed as fold induction over control unstimulated cells. Results combined from 2 independent experiments and represent 3 independent experiments. *p < 0.05, **p < 0.01 compared to LPS controls.
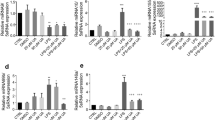
In order to assess the effect of oxymatrine on immune cell activity, we generated BMDCs from WT mice. Analysis of the kinetics of LPS-induced Il6 and Tnf mRNA accumulation in treated BMDCs showed that oxymatrine significantly inhibited Il6 at 1 and 4 hr (p < 0.01) and Tnfα at 4 hr of stimulation (p < 0.01), while Il1β expression was unaffected ( Fig. 2 ). These findings indicate that oxymatrine prevents expression in a selective group of LPS-induced pro-inflammatory genes in both IEC and immune cells.
Oxymatrine inhibits LPS-induced pro-inflammatory cytokine expression in BMDCs.
Naïve BMDCs were stimulated with 1 μg/mL LPS for 1 or 4 h ± 4 mg/mL OMT and real-time PCR performed to analyze Il6, Il1b and Tnfα mRNA accumulation standardized to Gapdh expression. Results are combined data from 3 independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 compared to LPS control.
Oxymatrine prevents NF-κB activity without blocking IκBα phosphorylation and through inhibition of p65 nuclear translocation
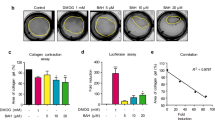
Since LPS-induced Cxcl2 is dependent on NF-κB activity16, we investigated the impact of oxymatrine on this pathway. Using an NF-κB luciferase reporter assay we were able to show that oxymatrine significantly inhibited LPS-induced NF-κB transcriptional activity in a dose-dependent manner compared to control LPS stimulated cells (p < 0.01) ( Fig. 3a ). To identify the target of oxymatrine mediated NF-κB blockade, we investigated the status of IκBα phosphorylation/degradation. As expected, we observed a time-dependent increase in LPS-induced IκBα phosphorylation with concomitant degradation of total IκBα ( Fig. 3b ). Interestingly, oxymatrine failed to prevent LPS-induced IκBα phosphorylation/degradation ( Fig. 3b ). Reduced IκBα levels in LPS-stimulated, oxymatrine-treated cells at 90 min likely reflects the canonical nature of NF-κB signaling where blockade of the transcription factor leads to decreased expression of its inhibitor. In contrast, oxymatrine significantly reduced (p < 0.05) LPS-induced p38MAPK phosphorylation in IECs at 60 min ( Fig. 3c ).
Oxymatrine inhibits NF-κB activation independent of IκBα phosphorylation.
(a) IEC-18 cells were transduced using an NF-κB luciferase construct and stimulated with 5 μg/mL LPS ± 4 mg/mL OMT. Luciferase production was measured and reported as fold induction over control normalized to amount of extract protein (light units/μg). Results are combined from 3 independent experiments. (b) Western blot analysis of phospho-IκBα and total IκBα was performed using IEC-18 cells stimulated with 5 μg/mL LPS for 30, 60 or 90 minutes ± 4 mg/mL OMT. β-Actin was used as a loading control. Densitometric analysis of protein levels is shown on the right side. Representative of 3 independent experiments. (c) Western blot analysis of phospho-p38, total p38 and β-Actin was performed using IEC-6 cells stimulated with LPS for 30 or 60 minutes ± OMT. Densitometric analysis of protein levels is shown on the right side. Representative of 3 independent experiments. **p < 0.01 compared to LPS control.
Because NF-κB transcriptional activity was blocked independently of IκBα phosphorylation/degradation by oxymatrine treatment, we investigated the subcellular compartmentalization of the NF-κB transcriptional subunit p65. IEC-6 cells were treated with oxymatrine and stimulated with LPS for 0–60 min and p65 localization determined by immunofluorescent microscopy. Pre-treatment with oxymatrine followed by LPS stimulation resulted in a statistically significant reduction in LPS-induced p65 nuclear accumulation compared to LPS-only treated cells (∼40%; p < 0.01) ( Fig. 4a–b ). Although higher oxymatrine concentration (8 mg/mL) resulted in an increased inhibition (∼70%) of nuclear translocation ( Supplementary Fig. S1 ), this dosage was associated with cellular toxicity (data not shown). To confirm that oxymatrine prevents LPS-induced NF-κB binding to the Cxcl2 promoter, we performed p65 chromatin immunoprecipitation using IEC-18 cells. LPS stimulation resulted in increased p65 binding to the Cxcl2 gene promoter at 30 min, which then decreased by 60 min, a process inhibited by oxymatrine treatment ( Fig. 4c ).
Oxymatrine inhibits p65 nuclear translocation.
(a) IEC-6 cells were treated with 5 μg/mL LPS ± 4 mg/mL OMT for 30 minutes. Cells were fixed, permeabilized and immunofluorescent staining performed for p65 (inner panels). Hoescht nuclear staining was also performed (outer panels). Representative of 3 independent experiments. (b) p65 cytosolic and nuclear quantifications were measured as p65 nuclear accumulation in 3 random fields of view per slide. Graph represents combined data from 3 independent experiments. (c) Chromatin immunoprecipitation (ChIP) analysis of p65 binding to the Cxcl2 promoter of IEC-18 cells stimulated with LPS ± OMT for 30 and 60 min. PCR amplification products were separated using agarose gel electrophoresis and gel scan results are presented in inverse color. Representative of 2 independent experiments. **P < 0.01 compared to LPS control.
Oxymatrine ameliorates DSS-induced colitis
To determine the potential in vivo anti-inflammatory therapeutic effect of oxymatrine, we tested this herbal product using the DSS model of acute intestinal inflammation. DSS-induced weight-loss was approximately 15% in control mice, whereas mice treated with low (50 mg/kg) or high (200 mg/kg) doses of oxymatrine were significantly protected from weight-loss by d 9 ( Fig. 5a ; p < 0.05). Oxymatrine-injected control groups displayed no significant change in body weight ( Fig. 5a ). Histological analysis revealed a significantly lower DSS injury score (p < 0.05) with either low- or high-dose oxymatrine treatment ( Fig. 5b–c ). We examined the levels of colonic inflammatory mediators in the different groups using real-time PCR and found a significant increase (p < 0.05) of colonic Il6, Il1β and Tnfα mRNA accumulation in DSS-exposed mice ( Fig. 5d ). Interestingly, DSS-induced Il6 and IL1β mRNA accumulation were dose-dependently inhibited in oxymatrine-treated mice ( Fig. 5d ). However, oxymatrine failed to inhibit DSS-induced TNF mRNA accumulation in vivo ( Fig. 5d ). In addition, expression of the proliferative marker Ki-67 within intact colonic crypts decreased in DSS-exposed, oxymatrine-treated mice compared to untreated mice, suggesting an improved intestinal response ( Fig. 5e , Supplementary Fig. S2 ). To gain more insight into in vivo NF-κB activation, we utilized NF-κBEGFP reporter mice17. We observed increased EGFP expression in DSS-exposed NF-κBEGFP mice compared to untreated mice ( Fig. 5f ). NF-κBEGFP mice exposed to DSS and treated with 200 mg/Kg oxymatrine showed similar pattern of EGFP expression to DSS-exposed mice ( Fig. 5f ) and these expression levels were confirmed using western blot analysis ( Supplementary Fig. S2 ). These findings showed that oxymatrine reduced DSS-induced acute colitis, which correlates with attenuation of a selective set of pro-inflammatory cytokine gene expression, without globally preventing NF-κB activation.
Oxymatrine ameliorates DSS-induced pathology.
WT C57BL/6 mice were pre-treated with saline or OMT for 2 days prior to addition of 3% DSS to drinking water. (a) Daily weights were measured (n = 5-7/group) and plotted as percentage bodyweight change from initial weight, expressed as group % mean ± SEM. (b) Representative colon swiss roll sections stained with H&E (original magnification 100X). (c) Group colon histopathological scores. Bars represent mean ± SEM. (d) Real-time PCR analysis of colonic tissue Tnf, Il6 and Il1b expression standardized to Gapdh expression. Expressed as fold induction over mock treated control mice. Representative of 2 independent experiments. (e) Immunhistochemical analysis of Ki-67 expression was performed using colon sections from control untreated, DSS-treated and DSS + 200 mg/Kg OMT-treated mice. Graph represents quantification of Ki-67+ cells within intact distal colonic crypts. 3 sections were stained and counted per condition. (f) Macroscopic analysis of representative colons from experimental NF-κBEGFP mouse groups, fluorescent analysis (right panel). Representative of 2 independent experiments. *p < 0.05, **p < 0.01 compared to DSS-only control.
Discussion
The increasing interest in alternative medicine worldwide, especially naturally derived herbs and plant extracts, has spurned research into elucidating mechanism of action attributed to these compounds. Oxymatrine has shown promising beneficial effects in rodent models of I/R-induced liver, heart and lung injury9,10,11. In this study, we show that oxymatrine administration is able to significantly inhibit LPS-induced pro-inflammatory cytokine production in both immune and non-immune cells. We demonstrate that this inhibitory effect is associated with decreased NF-κB p65 nuclear migration. Finally, we validated the therapeutic potential of oxymatrine in vivo by showing that DSS-induced acute injury and colitis was attenuated by both low and high doses of the natural product. It is important to note that the intestinal epithelial cell layer is severely damaged upon DSS exposure, which causes a massive influx of bacteria and bacterial products (including LPS) into the normally segregated mucosal immune system. Therefore, the use of LPS in vitro is a means to reproduce the secondary effect of DSS in vivo (exposure of IEC and immune cells to bacterial products). This standard model of LPS stimulation in vitro to simulate and measure inflammatory responses and the use of DSS-induced injury and colitis in vivo has been used in numerous reports18,19,20,21,22,23 including studies published from our group24,25.
An intriguing aspect of our study is that oxymatrine failed to inhibit LPS-induced IκBα phosphorylation/degradation, while blocking p65 nuclear translocation. This suggests that oxymatrine mediates its effect downstream of the classical IKK/IκB induced NF-κB activation. However, the p65 subunit contains a nuclear localization domain that is recognized by the karyopherin chaperone protein importin3α and interference between this chaperone protein and target p65 can impact nuclear shuttling26. Although we observed decreased p65/Importin α3 binding at steady state levels in the presence of oxymatrine, attempts to show increased interaction between the chaperone and p65 following LPS stimulation were unsuccessful (data not shown). Regardless of the exact mechanism of action, our study clearly demonstrates an inhibitory effect of oxymatrine on signal-induced p65 nuclear translocation at the level of protein localization (immunofluorescence) and DNA binding (ChIP assay). Previous studies by Fan H et al. showed that oxymatrine acts by reducing p65 protein expression in a rat model of TNBS-induced colitis14 and a recent report shows that matrine, the related herbal product derived from the same plant, inhibits NF-κB in neurons and astrocytes in an IκB-dependent manner27. However, as we failed to observe either decreased p65 expression in oxymatrine-treated cells or inhibition of IκB-phosphorylation, we conclude that oxymatrine's main mechanism of action is sequestration of p65 in the cytoplasm, at least in IECs and BMDCs.
Oxymatrine shows no apparent global inhibitory effect on NF-κB transcriptional activity in vivo as seen in oxymatrine-treated, DSS-exposed NF-κBEGFP mice. This is an important observation since NF-κB transcriptional activity is necessary to maintain epithelial barrier function and proper response to wound healing28,29,30,31. Though we see no global inhibition of NF-κB activity, mice treated with DSS and oxymatrine showed lower numbers of Ki-67+ cells within intact colonic crypts compared to untreated mice and far less than DSS treated mice. This difference may be indicative of the selective ability of oxymatrine to alter gene expression as we also observed a significant decrease in Il6 and Il1β mRNA levels but sustained levels of Tnfα mRNA in vivo. Interestingly, TNFα exerts a protective function in DSS-induced colitis32,33 and this may explain the beneficial impact of oxymatrine in vivo. Though oxymatrine blocks LPS-induced TNFα production in both IECs and BMDCs in vitro, the in vivo source of TNFα is not clear. Intestinal myofibroblasts34 and NKT35 cells are an important source of TNFα and could be unaffected by oxymatrine exposure. In addition, it is possible that the effect of oxymatrine on NF-κB in vivo could be coupled with effects on other signaling pathways such as p38MAPK11,12 and acts to balance target gene transactivation lowering pro-inflammatory effects while preserving cyto-protective ones. A more comprehensive analysis (RNA-seq, gene array) would help understand the impact of oxymatrine-mediated transcriptional inhibition.
Although oxymatrine demonstrates a beneficial effect in the DSS-model of acute intestinal injury, it should be noted that not all herbal products ameliorate colitis. We recently showed that while another herbal product tomato lycopene extract (TLE) proved effective in reducing LPS-induced NF-κB activity in vitro by blocking IκBα phosphorylation and p65 nuclear translocation, it exacerbated pathology in the DSS-induced injury model of colitis24. This deleterious effect correlated with a strong increase in colonic NF-κB (EGFP) activity in NF-κBEGFP mice, likely promoting expression of inflammatory cytokines by intestinal immune cells. Although TLE and oxymatrine both blocked NF-κB activity in vitro, the differential effects on colitis (protective vs deleterious) may be related to the extent of NF-κB inhibition. To that end, it is worth nothing that though the use of a higher oxymatrine concentration in vitro resulted in an increased inhibition of LPS-induced p65 nuclear translocation, this higher concentration was also associated with cellular toxicity. This suggests then that it is possible that NF-κB cyto-protective effect remains in oxymatrine-treated mice at the levels used in our study.
Although oxymatrine is considered to be safe and effective in treating Hepatitis B viral infections in China36,37, additional investigation would be needed before this natural product could be considered for clinical use in the United States for treating intestinal inflammatory disorders. It would be important to demonstrate the efficacy of oxymatrine using various experimental models of chronic colitis such as the T-cell transfer or genetically engineered mice. In summary, our findings demonstrate that oxymatrine does possess anti-inflammatory effects in vitro and in vivo, acting at least in part through partial inhibition of NF-κB p65 nuclear translocation, which is independent of IκBα phosphorylation/degradation.
Methods
Animal ethics statement
All animal procedures were performed in accordance with UNC institutional animal care and use committee (IACUC)-approved protocols. Mice were housed in specific pathogen-free (SPF) facilities at the University of North Carolina at Chapel Hill.
Cell culture and treatment
The non-transformed rat small intestinal cell lines IEC-6 (ATCC CRL 1592, American Tissue Culture Collection (ATCC), Manassas, VA) and IEC-18 (ATCC CRL 1589) were cultured as previously described38. IEC cells were plated in 6-well plates (2.5 × 105) or 10 cm tissue culture plates (1 × 106 cells/mL) for experiments. Bone marrow-derived dendritic cells (BMDCs) were generated from wild-type C57BL/6 mice as previously described39 and used at day-5 of culture for experiments. BMDCs were plated at a density of 1 × 106 cells/mL in 6-well plates.
All cells were washed with serum-free media and serum-starved for 2 hr prior to incubation with oxymatrine (98% purity, Narula Research, Chapel Hill, NC). Oxymatrine was reconstituted in saline and added to media for 1 hr prior to LPS stimulation. LPS (Escherichia coli serotype O111:B4, Sigma-Aldrich, St. Louis, MO) was used at various concentrations to stimulate IECs and BMDCs.
NF-κB luciferase reporter assay
IEC-18 cells were infected for 16 h with an adenoviral vector encoding an NF-κB-luciferase reporter gene (Ad5κB-LUC) as previously described40. Cells were pretreated with various concentrations of oxymatrine for 1 h, followed by stimulation with LPS (5 μg/mL) for 8 h. Cell extracts were prepared using luciferase cell lysis buffer (PharMingen, San Digeo, CA). Luciferase assays were performed using an LMax luminometer microplate reader (Molecular Devices, Sunnyvale, CA) and results were normalized for extract protein concentrations measured with the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA) as previously described41.
NF-κB immunofluorescence and Ki-67 immunohistochemistry
IEC-6 cells were pretreated with oxymatrine (4 mg/mL) for 1 hr and stimulated with LPS (5 μg/mL) for 30 min or 1 hr, cells were fixed and permeabilized using 100% ice-cold methanol for 10 min at 4°C. Immunofluorescent staining using p65 antibody (Millipore, anti-NF-κB CT, Billerica, MA) was performed as previously described38. Nuclear p65 aggregation was estimated by counting 3 random fields of view per slide utilizing an Olympus IX70 inverted epifluorescent microscope and data were reported as percentage of nuclear p65 positive cells.
Immunohistochemistry (IHC) was performed using tissue sections deparaffinized in xylene and rehydrated using a series of graded alcohol washes. Sections were probed using a Ki-67 monoclonal antibody (Dako, Carpinteria, CA) and biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) as previously described42.
Western blot analysis
Proteins were extracted with RIPA buffer and quantified using Bio-Rad protein assay (Bio-Rad). Proteins (20 μg/sample) were separated by SDS-PAGE and transferred to nitrocellulose membranes (Hybond-C Extra Amersham Biosciences, Piscataway, NJ). Antibodies to phospho-IκBα (Cell Signaling Technology, Danvers, MA), phospho-p38MAPK and total p38MAPK (Cell Signaling) were diluted 1∶500 in 5% bovine serum albumin/TBS-tween and antibodies to total IκBα (Cell Signaling) 1∶1000 and β-Actin (Santa Cruz Biotechnology, La Jolla, CA) 1∶10,000 in 5% milk/TBS-tween. Immunoreactive proteins were detected using the enhanced chemiluminescence light (ECL) detecting kit (Amersham, GE Healthcare Biosciences, Pittsburgh, PA) as previously described38. Densitometric values of immunoblot signals were obtained from 3 separate experiments using Adobe Photoshop.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitations were performed as previously described39,43. Briefly, IEC-18 cells were stimulated with LPS (5 μg/mL) alone or with oxymatrine (4 mg/mL) for 30 and 60 min and ChIP assay performed using a ChIP assay kit (Upstate-Cell Signaling Solutions, Temecula, CA) according to the manufacturer's specifications. Immunoprecipitation was carried out overnight with 2 μg of p65 (c-20; Santa Cruz Biotechnology) or RNA polymerase II (c-21; Santa Cruz Biotechnology) antibodies. PCR was performed with total input DNA (5 μl) and immunoprecipitated DNA (5 μl) using cxcl2 promoter-specific primers: CXCL2 promoter forward, 5′-CCTTCTTCCTGATGCAGGG-3′; CXCL2 promoter reverse, 5′-AGTCTGGGGCTGTGAGGTC-3′. Thermal cycle conditions were as follows: one cycle of 5 min of 94°C and 40 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 30 s followed by an extension of 7 min at 72°C. The PCR products were subjected to electrophoresis on 2% agarose gels containing GelStar fluorescent dye (Cambrex BioScience, Rockland, ME). Fluorescence staining was captured using an Alpha Imager 2000 (Alpha Innotech, Santa Clara, CA).
Reverse transcriptase PCR and real time PCR
RNA was isolated using Trizol reagent (Invitrogen, Grand Island, NY) and manufacturer's recommended protocol. cDNA was prepared using reverse-transcriptase amplification kit (Invitrogen) and cytokine expression quantitated using real-time PCR (Applied Biosystems 7900HT Fast Real-Time PCR System) and SYBR Green PCR Master Mix kit (Qiagen, Valencia, CA). Primer sequences for rat Cxcl-2, murine TNFα, IL-6, IL-1β and GAPDH primers were used as previously described24. Sequences for rat TNFα44, GAPDH45, IL-6 and IL-1β were also previously described46.
DSS colitis
8–12 week-old conventionally raised wild-type C57BL/6 mice or NF-κBEGFP mice (for NF-κB transcriptional studies) were exposed to 3% DSS (TdB Consultancy, Uppsala, Sweden) in drinking water as previously described47. Oxymatrine and saline solutions were prepared daily and sterilized using a 0.22 μm filter (Fisher Scientific, Pittsburg, PA) prior to intraperitoneal injection (i.p). Oxymatrine dosage (50 and 200 mg/kg) were selected based on previous in vivo studies8,9,10,12,14. Mice were injected with oxymatrine 2 days prior to exposure to DSS. Mice were monitored daily for weight loss as well as signs of rectal bleeding and diarrhea. At d 9 of DSS administration, mice were sacrificed, sections were taken from the distal, proximal colon and cecum for histological assessment. EGFP expression was imaged as described previously24. Mice were sacrificed at the end of DSS-exposure according to our IACUC-approved protocol and colons excised. Tissues were prepared for swiss-roll analysis as previously described47. Analysis of histopathology was determined using the scoring system developed by Cooper48 and Dieleman49 and modified by Williams50 using a 0 to 40 scale as previously described47.
Statistics
Data are represented as means ± SEM. Statistics were calculated using GraphPad Prism 5.0d software, utilizing non-parametric 2-tailed t tests, One-way ANOVA or Mann-Whitney analysis and considered significant if p values were <0.05.
References
Kamboj, V. P. Herbal medicine. Current Science 78, 35–39 (2000).
Bent, S. Herbal medicine in the United States: review of efficacy, safety and regulation: grand rounds at University of California, San Francisco Medical Center. J Gen Intern Med 23, 854–859 (2008).
Egan, B., Hodgkins, C., Shepherd, R., Timotijevic, L. & Raats, M. An overview of consumer attitudes and beliefs about plant food supplements. Food Funct 2, 747–752 (2011).
Barnes, P. M., Powell-Griner, E., McFann, K. & Nahin, R. L. Complementary and alternative medicine use among adults: United States, 2002. Adv Data 1–19 (2004).
Ritchie, M. R. Use of herbal supplements and nutritional supplements in the UK: what do we know about their pattern of usage? Proc Nutr Soc 66, 479–482 (2007).
Cui, X., Wang, Y., Kokudo, N., Fang, D. & Tang, W. Traditional Chinese medicine and related active compounds against hepatitis B virus infection. Biosci Trends 4, 39–47 (2010).
Wang, Y.-P. et al. Oxymatrine inhibits hepatitis B infection with an advantage of overcoming drug-resistance. Antiviral Research 89, 227–231 (2011).
Deng, Z.-Y., Li, J., Jin, Y., Chen, X.-L. & Lü, X.-W. Effect of oxymatrine on the p38 mitogen-activated protein kinases signalling pathway in rats with CCl4 induced hepatic fibrosis. Chin. Med. J. 122, 1449–1454 (2009).
Jiang, H., Meng, F., Li, J. & Sun, X. Anti-apoptosis Effects of Oxymatrine Protect the Liver from Warm Ischemia Reperfusion Injury in Rats. World J. Surg. 29, 1397–1401 (2005).
Hong-li, S. et al. Cardioprotective effects and underlying mechanisms of oxymatrine against Ischemic myocardial injuries of rats. Phytother. Res. 22, 985–989 (2008).
Zhao, J. et al. Oxymatrine attenuates intestinal ischemia/reperfusion injury in rats. Surg Today 38, 931–937 (2008).
Xu, G. L. et al. Attenuation of acute lung injury in mice by oxymatrine is associated with inhibition of phosphorylated p38 mitogen-activated protein kinase. Journal of Ethnopharmacology 98, 177–183 (2005).
Liu, Y., Zhang, X.-J., Yang, C.-H. & Fan, H.-G. Oxymatrine protects rat brains against permanent focal ischemia and downregulates NF-κB expression. 1268, 174–180 (2009).
Fan, H. et al. Oxymatrine improves TNBS-induced colitis in rats by inhibiting the expression of NF-κB p65. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 28, 415–420 (2008).
Zheng, P., Niu, F.-L., Liu, W.-Z., Shi, Y. & Lu, L.-G. Anti-inflammatory mechanism of oxymatrine in dextran sulfate sodium-induced colitis of rats. World J. Gastroenterol. 11, 4912–4915 (2005).
Widmer, U., Manogue, K. R., Cerami, A. & Sherry, B. Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1 alpha and MIP-1 beta, members of the chemokine superfamily of proinflammatory cytokines. J. Immunol. 150, 4996–5012 (1993).
Magness, S., Jijon, H. & Fisher, N. In vivo pattern of lipopolysaccharide and anti-CD3-induced NF-κB activation using a novel gene-targeted enhanced GFP reporter gene mouse. J. Immunol. 173, 1561–1570 (2004).
Wen, T. et al. A novel tylophorine analog NK-007 ameliorates colitis through inhibition of innate immune response. International Immunopharmacology (2012). doi:10.1016/j.intimp.2012.08.008.
Liu, Y.-W., Su, Y.-W., Ong, W.-K., Cheng, T.-H. & Tsai, Y.-C. Oral administration of Lactobacillus plantarum K68 ameliorates DSS-induced ulcerative colitis in BALB/c mice via the anti-inflammatory and immunomodulatory activities. International Immunopharmacology 11, 2159–2166 (2011).
Fiorotto, R. et al. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-κB-mediated inflammatory response in mice. Gastroenterology 141, 1498–508–1508. e1–5 (2011).
Han, E. S., Oh, J. Y. & Park, H.-J. Cordyceps militaris extract suppresses dextran sodium sulfate-induced acute colitis in mice and production of inflammatory mediators from macrophages and mast cells. Journal of Ethnopharmacology 134, 703–710 (2011).
Dutra, R. C. et al. Preventive and therapeutic euphol treatment attenuates experimental colitis in mice. PLoS ONE 6, e27122 (2011).
Yoshino, T. et al. Immunosuppressive effects of tacrolimus on macrophages ameliorate experimental colitis. Inflamm Bowel Dis 16, 2022–2033 (2010).
Joo, Y.-E. et al. Tomato lycopene extract prevents lipopolysaccharide-induced NF-kappaB signaling but worsens dextran sulfate sodium-induced colitis in NF-kappaBEGFP mice. PLoS ONE 4, 1–11 (2009).
Larmonier, C. B. et al. Limited effects of dietary curcumin on Th-1 driven colitis in IL-10 deficient mice suggest an IL-10-dependent mechanism of protection. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G1079–91 (2008).
Theiss, A., Jenkins, A. & Okoro, N. Prohibitin Inhibits TNF alpha–induced NFkB Nuclear Translocation via the Novel Mechanism of Decreasing Importin-a3 Expression. Mol. Biol. Cell 20, 4412–4423 (2009).
Xu, M., Yang, L., Hong, L.-Z., Zhao, X.-Y. & Zhang, H.-L. Direct protection of neurons and astrocytes by matrine via inhibition of the NF-κB signaling pathway contributes to neuroprotection against focal cerebral ischemia. 1454, 48–64 (2012).
Fox, J., Rogers, A., Whary, M. & Ge, Z. Gastroenteritis in NF-κB-deficient mice is produced with wild-type Camplyobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect. Immun. 72, 1116–1125 (2004).
Zaph, C. et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature 446, 552–556 (2007).
Lebeis, S., Bommarius, B. & Parkos, C. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J. Immunol. 179, 566–577 (2007).
Reuther-Madrid, J. Y. et al. The p65/RelA subunit of NF-kappaB suppresses the sustained, antiapoptotic activity of Jun kinase induced by tumor necrosis factor. Mol. Cell. Biol. 22, 8175–8183 (2002).
Stillie, R. & Stadnyk, A. W. Role of TNF receptors, TNFR1 and TNFR2, in dextran sodium sulfate-induced colitis. Inflamm Bowel Dis 15, 1515–1525 (2009).
Naito, Y. et al. Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J. Gastroenterol. Hepatol. 18, 560–569 (2003).
Di Sabatino, A. et al. Functional modulation of Crohn's disease myofibroblasts by anti-tumor necrosis factor antibodies. Gastroenterology 133, 137–149 (2007).
Singh, U. P. et al. CXCL10+ T cells and NK cells assist in the recruitment and activation of CXCR3+ and CXCL11+ leukocytes during Mycobacteria-enhanced colitis. BMC Immunology 9, 25 (2008).
Lu, L., Zeng, M., Mao, Y. & Wan, M. Oxymatrine in the treatment of chronic hepatitis B for one year: a multicenter random double-blind placebo-controlled trial]. Zhonghua Gan Zang Bing Za Zhi 12, 597–600 (2004).
Lu, L., Zeng, M., Mao, Y., Li, J. & Wan, M. Oxymatrine therapy for chronic hepatitis B: a randomized double-blind and placebo-controlled multi-center trial. World Journal of Gastroenterology 9, 2480–2483 (2003).
Jobin, C., Haskill, S., Mayer, L., Panja, A. & Sartor, R. B. Evidence for altered regulation of I kappa B alpha degradation in human colonic epithelial cells. J. Immunol. 158, 226–234 (1997).
Mühlbauer, M., Chilton, P. M., Mitchell, T. C. & Jobin, C. Impaired Bcl3 up-regulation leads to enhanced lipopolysaccharide-induced interleukin (IL)-23P19 gene expression in IL-10(-/-) mice. J. Biol. Chem. 283, 14182–14189 (2008).
Haller, D. IKKbeta and Phosphatidylinositol 3-Kinase/Akt Participate in Non-pathogenic Gram-negative Enteric Bacteria-induced RelA Phosphorylation and NF-kappa B Activation in Both Primary and Intestinal Epithelial Cell Lines. Journal of Biological Chemistry 277, 38168–38178 (2002).
Kim, J.-S. & Jobin, C. The flavonoid luteolin prevents lipopolysaccharide-induced NF-kappaB signalling and gene expression by blocking IkappaB kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Immunology 115, 375–387 (2005).
Uronis, J. M. et al. Modulation of the Intestinal Microbiota Alters Colitis-Associated Colorectal Cancer Susceptibility. PLoS ONE 4, e6026 (2009).
Hoentjen, F., Sartor, R. B., Ozaki, M. & Jobin, C. STAT3 regulates NF-kappaB recruitment to the IL-12p40 promoter in dendritic cells. Blood 105, 689–696 (2005).
Wang, X., Li, X., Erhardt, J. A., Barone, F. C. & Feuerstein, G. Z. Detection of tumor necrosis factor-alpha mRNA induction in ischemic brain tolerance by means of real-time polymerase chain reaction. J. Cereb. Blood Flow Metab. 20, 15–20 (2000).
Karrasch, T., Spaeth, T., Allard, B. & Jobin, C. PI3K-dependent GSK3ß(Ser9)-phosphorylation is implicated in the intestinal epithelial cell wound-healing response. PLoS ONE 6, 1–9 (2011).
Tanga, F. Y., Raghavendra, V. & DeLeo, J. A. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem. Int. 45, 397–407 (2004).
Karrasch, T., Kim, J.-S., Jang, B. I. & Jobin, C. The flavonoid luteolin worsens chemical-induced colitis in NF-kappaB(EGFP) transgenic mice through blockade of NF-kappaB-dependent protective molecules. PLoS ONE 2, 1–15 (2007).
Cooper, H., Murthy, S. & Shah, R. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 69, 238–249 (1993).
Rees, V. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114, 385–391 (1998).
Williams, K., Fuller, C. R. & Dieleman, L. Enhanced survival and mucosal repair after dextran sodium sulfate-induced colitis in transgenic mice that overexpress growth hormone2. Gastroenterology 120, 925–937 (2001).
Acknowledgements
We thank AN for the suggestion to investigate oxymatrine. Histology was performed at the Center for Gastrointestinal Biology & Disease histology core (P30 DK034987). This work was supported by the National Institutes of Health R01DK047700 and R01DK073338 to C.J, Training, Workforce Development and Diversity division of the National Institute of General Medical Sciences (NIGMS) grant K12GM000678 to J.R.G.
Author information
Authors and Affiliations
Contributions
The co-first authors JRG and JSK were responsible for primary data generation, analysis and writing of the manuscript. Co-author JRG and MM were involved in in vivo experimentation, immunofluorescent studies and assessment of colitis. AN provided the source of oxymatrine and CJ is the principal investigator and corresponding author for these studies.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplemental Data
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Guzman, J., Koo, J., Goldsmith, J. et al. Oxymatrine Prevents NF-κB Nuclear Translocation And Ameliorates Acute Intestinal Inflammation. Sci Rep 3, 1629 (2013). https://doi.org/10.1038/srep01629
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01629
This article is cited by
-
Role of Rho GTPases in inflammatory bowel disease
Cell Death Discovery (2023)
-
Involvement of LARP7 in Activation of SIRT1 to Inhibit NF-κB Signaling Protects Microglia from Acrylamide-Induced Neuroinflammation
Neurotoxicity Research (2022)
-
Protective effect of Oxymatrine against acute spinal cord injury in rats via modulating oxidative stress, inflammation and apoptosis
Metabolic Brain Disease (2020)
-
Inhibitory effects of oxymatrine on hepatic stellate cells activation through TGF-β/miR-195/Smad signaling pathway
BMC Complementary and Alternative Medicine (2019)
-
Matrine inhibits the development and progression of ovarian cancer by repressing cancer associated phosphorylation signaling pathways
Cell Death & Disease (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.