Abstract
Cigarette smoking contributes to the development of destructive periodontal diseases and delays its healing process. Our previous study demonstrated that nicotine, a major constituent in the cigarette smoke, inhibits the regenerative potentials of human periodontal ligament-derived stem cells (PDLSC) through microRNA (miRNA) regulation. In this study, we hypothesized that the delayed healing in cigarette smokers is caused by the afflicted regenerative potential of smoker PDLSC. We cultured PDLSC from teeth extracted from smokers and non-smokers. In smoker PDLSC, we found significantly reduced proliferation rate and retarded migration capabilities. Moreover, alkaline phosphatase activity, calcium deposition and acidic polysaccharide staining were reduced after BMP2-induced differentiation. In contrast, more lipid deposition was observed in adipogenic-induced smoker PDLSC. Furthermore, two nicotine-related miRNAs, hsa-miR-1305 (22.08 folds, p = 0.040) and hsa-miR-18b (15.56 folds, p = 0.018), were significantly upregulated in smoker PDLSC, suggesting these miRNAs might play an important role in the deteriorative effects on stem cells by cigarette smoke. Results of this study provide further evidences that cigarette smoking affects the regenerative potentials of human adult stem cells.
Similar content being viewed by others
Introduction
Cigarette smoking is a major contributing factor to the death from cancer, cardiovascular diseases and pulmonary diseases1. This is a serious health problem worldwide as the prevalence of cigarette smoking among adults is still high2,3. The first exposure of cigarette smoke is experienced by the oral tissues and its chemical components are rapidly absorbed and circulated in the human bloodstream. Not only influencing the systemic organs, cigarette smoking can also leads to deterioration of periodontal conditions and the development of destructive periodontal diseases4. The fundamental principle of regenerative therapy against periodontal diseases, which is characterized by loss of connective tissue and bone support, is to stimulate the periodontal ligament (PDL) cells and to restore the PDL tissue5. However, most of the current treatments, such as the bone grafts, bone substitutes, barrier membrane or bioactive factors6, are not considered as regenerative techniques. Reliable strategy to form PDL-like structures is critical in regenerative medicine7. Recently, stem cell engineering offers great promises8.
Stem cells derived from PDL tissue (PDLSC) are adult resident tissue-restricted stem cells, participating in tissue maintenance and regeneration9. PDLSC contain a heterogeneous population of mesenchymal stem cells (MSC) and neural crest-derived stem cells. They are multipotent and capable of differentiating into the neurogenic, cardiomyogenic, chondrogenic and osteogenic lineages10,11,12. PDLSC enhance the disease healing process13. However, cigarette smoking has been found to delay this healing process14. Besides, the incidence of delayed healing is double in smokers than in non-smokers15, likely due to the inhibition of stem cell recruitment to tissues and regeneration by cigarette smoking16,17. Active components in cigarette smoke will influence these stem cell regenerative potentials and lead to delayed healing phenomena18. We previously showed that nicotine will affect the biophysical properties of human MSC, rendering the cells less responsive to mechano-induction and other physical stimuli19. Recently, we further demonstrated that nicotine inhibits the proliferation, migration and osteogenic differentiation of human MSC and PDLSC by changing their microRNA (miRNA) expression profiles20. The finding provides further evidence that active components in cigarette smoke can alter stem cell properties and affect their response to tissue regeneration.
Although nicotine is a key component out of the 3,500 chemicals in cigarette smoke21, stem cells exposed to nicotine in culture might not truly reflect the endogenous properties of stem cells from cigarette smokers. This can be resolved by extracting stem cells directly from teeth extracted from cigarette smokers. Here, we hypothesized that the delayed healing in cigarette smokers is the outcome from its effects on the regenerative potential of the smoker PDLSC through genetic and microRNA (miRNA) regulation. In this study, we determined the effect of cell proliferation, cell migration, osteogenic and adipogenic differentiation potential as well as miRNA expression of human PDLSC from cigarette smokers.
Results
Decreased proliferation activity in PDLSC from smokers
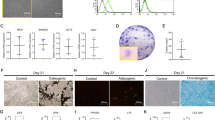
The availability and abundance of stem cells to exert regenerative effects was determined by the cell proliferative rates. In this study, all 6 primary cultures attained 70% confluence within 2 weeks of the first appearance of cell colony. They were expanded for 2 to 3 passages and cryopreserved. However, the proliferation of PDLSC from smokers was significantly slower than those of non-smokers by 2.53 folds at Day 5 (p = 0.006) and 2.88 folds at Day 7 (p = 0.022; Figure 1A). The increasing rate of PDLSC from non-smoker was higher than that from smokers throughout the testing period, indicating the loss of proliferative potential by cigarette smoking. The decreased proliferation rate of smoker PDLSC might not be related to the expression levels of MYC, KLF4, NOTCH1 and PCNA genes since they did not show statistical significant differences between smoker PDLSC and non-smoker PDLSC (Figure 1B).
Cell proliferation and gene expression analyses of human PDLSC from smokers and non-smokers.
(A) The proliferation of human PDLSC from smokers and non-smokers was measured by MTT assay for 7 days. The data represented was the mean of 3 samples ± standard deviation. Solid line: the smoker group; Dotted line: the non-smoker group. ‘*’: p < 0.05, ‘**’: p < 0.01. (B) The gene expression analysis was performed using the Sybr green PCR master mix on a real-time PCR machine. Genes related to stem cell markers (KLF4 and NOTCH1) and proliferation markers (MYC and PCNA) were examined. GAPDH was used for normalization. The data represented was the mean ± standard deviation.
Retarded migration capability in PDLSC from smokers
The movement of stem cells and their capacity to migrate to injury sites also determine regenerative effects. In our cell migration analysis, by the scratch wound assay, smoker PDLSC moved slower than the non-smoker PDLSC (Figure 2A). The reduction of scratch wound area by smoker PDLSC was also smaller than that by non-smoker PDLSC at 12 hours (20.07 ± 3.78% versus 25.92 ± 4.00%, p = 0.045) and at 24 hours (60.10 ± 8.55% versus 72.27 ± 5.90%, p = 0.031; Figure 2B). This suggested that cigarette smoking reduces the migration ability of PDLSC.
Cell migration analysis of human PDLSC from smokers and non-smokers.
(A) Migration of PDLSC from smokers and non-smokers was evaluated by scratch wound assay. Images were taken at time 0, 4, 8, 12 and 24 hours. Scale bar: 200 μm. (B) Area of reduction was measured by Image J software. The percentage migration was calculated by the average area reduction at 4, 8, 12 or 24-hour as compared to time 0. The data represented was the mean of 3 samples ± standard deviation. Solid line: the smoker group; Dotted line: the non-smoker group. ‘*’: p < 0.05.
Altered BMP2-induced and adipogenic differentiation potential in PDLSC from smokers
The ability of stem cells to differentiate into a designated mature cell type is the basis of stem cell regeneration. Under BMP-2 stimulation, PDLSC could differentiate into osteoblasts as the blue/purple precipitates indicating alkaline phosphatase activity increased (Figure 3A). However, the amount of blue/purple stain was reduced in PDLSC from smokers. Coherently, red stain of calcium deposition was accumulated in the cells under BMP2-induced differentiation (Figure 3B). Reduced calcium deposition was observed in smoker PDLSC. In addition, BMP2-treated PDLSC could also differentiate into chondrocytes as indicated by the blue stain of acidic polysaccharides (Figure 3C). Analogously, there was a slight reduction of the blue stain in smoker PDLSC after the BMP-2 treatment. PDLSC could also differentiate into adipocytes upon adipogenic induction as shown by the red stain for lipid (Figure 3D). In contrast, more lipid staining was observed in smoker PDLSC. These findings demonstrated that the differentiation potential of PDLSC into osteoblasts and chondrocytes was inhibited by cigarette smoking, but the differentiation into adipocytes was enhanced.
Alkaline phosphatase, calcium deposition, acidic polysaccharide and lipid analyses of human PDLSC from smokers and non-smokers upon BMP2-induced and adipogenic differentiation.
PDLSC were treated with BMP2-induced differentiation medium (culture medium supplemented with 10 μM β-glycerophosphate, 10 nM dexamethasone, 50 ng/ml BMP-2 and 50 μg/ml ascorbic acid) or adipogenic differentiation medium (culture medium supplemented with 1 μM indomethacin, 500 μM 3-isobutyl-1-methylxanthine, 1 μM dexamethasone and 10 μg/ml insulin) for 14 days. (A) Alkaline phosphatase activity was assessed by Burstone's staining protocol with NBT/BCIP reagent. (B) Calcium deposition was evaluated by Alizarin Red S staining. (C) Acidic polysaccharide was analysed by Alcian blue 8GX staining. (D) Lipid was evaluated by Oil Red O staining and nuclei were counter-stained by hematoxylin. Scale bar: 200 μm.
microRNA expression in PDLSC from smokers
To explore the effects of cigarette smoking on miRNA, the top 10 differentially expressed miRNAs from our previous miRNA microarray analysis in PDLSC after nicotine treatment were selected20: hsa-miR-18b, hsa-miR-30d, hsa-miR-137, hsa-miR-374b, hsa-miR-505, hsa-miR-1305, hsa-miR-1914*, hsa-miR-1973, hsa-miR-3198 and hsa-miR-3659. Compared to the non-smoker PDLSC, 3 out of 10 selected miRNAs were differentially expressed in smoker PDLSC (Table 1). Among them, 2 were upregulated (hsa-miR-1305: 22.08 folds, p = 0.040 and hsa-miR-18b: 15.56 folds, p = 0.018) and they showed the same trend with the expression in the nicotine-treated PDLSC. One miRNA (hsa-miR-3198: 42.98 folds, p = 0.016) was downregulated in smoker PDLSC. hsa-miR-1305 and hsa-miR-18b showed the same upregulated trend with the expression in the nicotine-treated PDLSC.
Target gene lists of miRNAs, which were differentially expressed and in the same trend with the nicotine treatment (629 genes for hsa-miR-1305 and 202 genes for hsa-miR-18b) were generated by the TargetScan in the GeneSpring software (Agilent). Gene ontology of the gene lists, analysed by DAVID (597 DAVID identities for hsa-miR-1305 and 190 DAVID identities for hsa-miR-18b), revealed that smoking-associated miRNAs (hsa-miR-1305 and hsa-miR-18b) might target the genes involved in cell cycle, cell projection, cell junction and cytoskeleton (Table 2), which further suggested that cigarette smoking would have influences on PDLSC proliferation and migration potential.
Discussion
Cigarette smoking has long been suggested as a potent risk factor for periodontal diseases22. Smokers were less responsive to periodontal therapy than non-smokers23. Cigarette smoking delays healing process14,15 and impairs inflammatory and immune responses to periodontal pathogens, exerting both systemic and local effects24. However, the mechanisms of cigarette smoking on periodontal diseases are still unclear. As residential stem cells are known to actively participate in tissue regeneration9, the delayed healing in cigarette smokers could be due to the afflicted PDLSC by cigarette smoke. Previous reports studied the exposure of nicotine on non-smoker PDLSC18,20,25. This study, for the first time, investigated the regenerative potential of PDLSC directly from cigarette smokers.
Stem cell regeneration is determined by 3 critical processes: cell proliferation, migration and differentiation26. Cell proliferation determines the amount of stem cells present within the body capable of exerting a regenerative effect. Smoking has been shown to decrease the level of circulating CD34+ progenitor cells in blood27. This reduced level could be caused by reduction in cell proliferative rates. The proliferation of osteoprogenitor cells is inhibited by the exposure to cigarette smoke extract28. Moreover, nicotine, the major constituent in the cigarette smoke, also inhibits cell proliferation in PDLSC20. Our results further proved that the proliferation rate of PDLSC from smokers is decreased when compared to that from non-smokers (Figure 1A). At Day 3, the proliferation of PDLSC from non-smokers is 1.79 folds higher than that from smokers. At Day 5, the fold difference is 2.53, whereas at Day 7 is 2.88 folds. It is notable that residential PDLSC are still present in smokers as we could establish the stem cell lines from smokers' PDL tissues. Hence, the reduced proliferation of stem cells from cigarette smokers led to a reduction in available adult stem cells. However, the differential proliferative power between smoker and non-smoker PDLSC might not be associated with the expression level of MYC, KLF4, NOTCH1 and PCNA genes (Figure 1B).
Cell migration allows stem cells to actively move towards the injury sites and contribute to the healing process29. Cigarette smoke extract inhibits chemotaxis of human osteoprogenitor cells30. Moreover, nicotine inhibits the migration of the PDL fibroblast31 as well as PDLSC20. This is coherent to the reduced migration ability of smoker PDLSC as evidenced in this study (Figure 2). The reduced migration of smoker PDLSC might be related to the downregulation of focal adhesion kinase expression as shown in the nicotine-treated PDLSC20,32. Further investigation is needed to confirm the proposition.
Stem cell differentiation into tissue-specific cells is one of the mechanisms for stem cell repair at the injury site during the healing process13,33. Cigarette smoke extract inhibits osteogenic differentiation of human osteoprogenitor cells28 and nicotine also reduces osteogenic differentiation of PDLSC20. Analogously, in this study, we showed that the calcium deposition, the alkaline phosphatase activity and acidic polysaccharides were reduced in PDLSC from smokers after BMP-2-induced differentiation (Figure 3A–3C). In contrast, the amount of lipid staining was increased in smoker PDLSC after adipogenic differentiation (Figure 3D). Enhanced adipogenic differentiation by cigarette smoke extract was also observed in Graves' orbital fibroblast34. Cigarette smoking could affect the differentiation potential of human PDLSC, which might be related to the delayed healing processes seen clinically.
In our previous study, we reported the first global miRNA expression profile of nicotine-treated PDLSC20. We identified 16 differentially expressed miRNAs in the nicotine-treated PDLSC. 12 of them (hsa-miR-7, hsa-miR-18b, hsa-miR-30d, hsa-miR-137, hsa-miR-374b, hsa-miR-505, hsa-miR-543, hsa-miR-1305, hsa-miR-1914*, hsa-miR-1973, hsa-miR-3198 and hsa-miR-3659) were upregulated and 4 of them (hsa-miR-210, hsa-miR-762, hsa-miR-1915 and hsa-miR-4281) downregulated. All of these miRNAs showed dose-dependent changes from 0.5 to 1.0 μM nicotine. In this study, we selected the top 10 miRNA with highest fold changes from the miRNA profile of nicotine-treated PDLSC for miRNA expression analysis in smoker PDLSC. hsa-miR-1305 and hsa-miR-18b were upregulated in smoker PDLSC and they showed the same upregulated trend with the expression in the nicotine-treated PDLSC (Table 1). Therefore, the reduced regenerative potentials of PDLSC from smokers could be regulated by these two miRNAs. In contrast, the expression trend of hsa-miR-3198 is in reverse to the expression in the nicotine-treated PDLSC, indicating that hsa-miR-3198 may not be responsible for the reduced regenerative potential in smoker PDLSC. Moreover, the predicted target genes of hsa-miR-1305 and hsa-miR-18b could be involved in regulating cell cycle, cell projection, cell junction and cytoskeleton (Table 2), which further suggested that cigarette smoking influenced PDLSC proliferation and migration potential. The differentially expressed miRNAs and their related target genes in this study should be further investigated for identifying the biological pathways affected by cigarette smoking in relation to reduced regenerative potentials on stem cells.
The biological function of hsa-miR-1305 in PDLSC is not known. In our previous study, hsa-miR-1305 target genes (PTK2 and RUNX2) were downregulated in nicotine-treated human PDLSC20. Upregulation of hsa-miR-1305 could be associated with downregulation of PTK2 and RUNX2, which affect stem cell migration and osteogenic differentiation29,35. The role of hsa-miR-18b in PDLSC is also unclear. Ectopic expression of miR-18b inhibited TGF-β1-induced differentiation of hair follicle stem cells into smooth muscle cells36. However, ectopic inhibition of miR-18b suppressed the migration of breast cancer cells37. Therefore, upregulation of hsa-miR-18b might lead to the reduced differentiation potentials, but not migration, in smoker PDLSC. The biological roles of these 2 miRNAs in stem cell regenerative functions as well as the in vivo effect of cigarette smoking on PDLSC regenerative potentials are needed to be investigated in further studies.
In summary, this study showed that the proliferation, migration and differentiation abilities of human PDLSC from smokers were altered. We also revealed altered miRNA expressions in smoker PDLSC, further providing evidence that miRNAs are a key regulator in these cigarette smoking-associated functional changes. This study provided the possible mechanistic explanations on stem cell-associated healing delay in cigarette smoking.
Methods
Teeth collection and PDLSC culture
Human permanent teeth were collected from 3 cigarette smokers (33 ± 7.21 years) and 3 age-matched non-smokers (32 ± 6.56 years; p = 0.868) undergoing routine extraction for orthodontic reasons at the Oral-Maxillofacial Surgery and Dental Unit, North District Hospital, Hong Kong. According to the standard current smoking definition of the National Survey on Drug Use and Health (NSDUH), cigarette smoker is defined as the subject with cigarette smoking during the past 30 days. This study was approved by the Ethics Committee of Department of Health, Hong Kong (L/M 118/2011), which is in accordance with the tenets of the Declaration of Helsinki. All donors gave written informed consent. Human PDLSC lines were established same as previous approach10,11. These cells were cultured in Dulbecco's modified Eagle's medium (Gibco BRL, Rockville, MD) supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL). The medium was changed every 2 days. Cells at passage 3-5 were used for the experiments. The stem cell properties, including stem cell marker expression and differentiation abilities, of our PDLSC lines have been reported previously.10,11,12,19,20,38,39
Cell proliferation analysis
The proliferation of PDLSC was evaluated by MTT (3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide) assay (Invitrogen, Carlsbad, CA). 1,000 cells per well were seeded on a 24-well plate (Corning Life Sciences, Lowell, MA) and the cells were cultured in regular medium for 7 days. The analysis (3 wells per sample) was performed on Day 0, 1, 3, 5 and 7. Briefly, each sample was incubated with 0.05 mg/ml MTT reagent for 3 hours. After washing out the excessive MTT reagent, the purple precipitates were dissolved by isopropanol and transferred to a 96-well plate (Corning Life Sciences) for intensity measurement. The absorbance at wavelength 570 nm with reference 650 nm was measured by a plate reader (Powerwave XS, Bio-Tek Instruments).
Cell migration analysis
PDLSC (1 × 105 cells per well) were seeded on a 12-well plate (Corning Life Sciences). Scratch wounds were created with 200-μl pipette tips on the pre-seeded confluence cells. The culture was washed after scratch wound induction and replaced by fresh serum-free medium. Photomicrographs were taken at time 0 (immediately following the scratch wound), 4, 8, 12 and 24 hours. The wound gaps were measured by ImageJ (version 1.47; NIH, Bethesda, MD). The percentage migration was calculated by the average area reduction at 4, 8, 12 or 24-hour as compared to time 0. Every well have 6 scratch wounds and triplicates performed for each sample.
BMP2-induced and adipogenic differentiation analysis
BMP2-induced differentiation medium was composed of 10 μM β-glycerophosphate (Sigma-Aldrich, St. Louis, MO), 10 nM dexamethasone (Sigma-Aldrich), 50 ng/ml BMP-2 (PeproTech, Rocky Hill, NJ) and 50 μg/ml ascorbic acid (Sigma-Aldrich) in the culture medium. PDLSC (5,000 cells per well) were seeded on the 8-well chamber slides (Thermo Scientific, Rochester, NY). The cells were treated with BMP2-induced differentiation medium for 14 days. For alkaline phosphatase activity, the cells were fixed in ice cool acetone and evaluated by Burstone's staining protocol with NBT/BCIP reagent (Roche, Indianapolis, IN). For calcium deposition, the cells were fixed in 10% formalin and evaluated by Alizarin Red S staining (Sigma-Aldrich). Since previous studies suggested that BMP2 also induces chondrogenic differentiation40,41, the presence of acidic polysaccharides was also analysed in the BMP2-treated PDLSC, which the cells were fixed in 10% formalin and evaluated by Alcian blue 8GX staining (Sigma-Aldrich).
Adipogenic differentiation medium was composed of 1 μM indomethacin (Sigma-Aldrich), 500 μM 3-isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich), 100 nM dexamethasone (Sigma-Aldrich) and 10 μg/ml insulin (PeproTech). PDLSC (5,000 cells per well) were seeded on the 8-well chamber slides (Thermo Scientific). The cells were treated with adipogenic differentiation medium for 14 days. After treatment, the cells were fixed in 10% formalin and stained Oil Red O staining (Sigma-Aldrich) for adipogenic differentiation analysis.
MicroRNA expression analysis
Human PDLSC at passage 3 was cultured with regular medium in 75 cm2 flask (Corning Life Sciences). Total RNA, including the miRNA fraction, was extracted by TRIzol reagent based on the manufacturer's protocol (Invitrogen). RNA concentrations were measured by Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE). Candidate miRNAs were selected from the top 10 differentially expressed miRNAs with highest fold-changes in our previous nicotine-treated PDLSC miRNA profile20. In semi-quantitative PCR of their expressions, total RNA (20 ng) was reverse transcribed by the Taqman MicroRNA Reverse Transcriptase kit (Applied Biosystems, Forster City, CA). The resultant products were quantified using the appropriate Taqman MicroRNA Assays (Applied Biosystems) on the LightCycler® 480 System (Roche). Results were normalized to U6 expression. The threshold cycle, Cp, was calculated by the second derivative maximum method. The fold change was determined by comparing to the non-smoking group with the ΔΔCT-method. Triplicates were performed for each sample.
Downstream mRNA targets of the miRNAs were predicted by TargetScan (http://genes.mit.edu/targetscan/index.html) in the GeneSpring GX 11.5 software (Agilent). Context percentile of 95 was the criterion for target prediction. DAVID Bioinformatics Resources 6.7 (http://david.abcc.ncifcrf.gov/)42 was used for gene enrichment and gene ontology analysis. Enrichment score greater than 1.3 was considered as significant.
Gene expression analysis
In order to further examine the stemness and proliferation ability of the PDLSC from smokers and non-smokers, we evaluate the gene expression of stem cell markers (KLF4 and NOTCH1) and proliferative marker (MYC and PCNA)11,43. cDNA was synthesized via reverse transcription of 1 μg total RNA using the Superscript III RT-PCR kit (Invitrogen) with random primers. The gene expression analysis was performed using the Sybr green PCR master mix (Applied Biosystems) with specific primers (Supplementary Table 1) on a real-time PCR machine (PRISM 7900HT Sequence Detection System; Applied Biosystems). Housekeeping gene (GAPDH) was used for normalization. Triplicates were performed for each sample. The relative expression levels were compared to the non-smoking group.
Statistical analysis
All statistical analyses were performed by commercially available software (IBM SPSS Statistics 20; SPSS Inc., Chicago, IL). Mean was obtained from the results of the 3 samples for each group. Independent T-test was used to compare the means between smoking and non-smoking groups. Significance was defined as p < 0.05.
References
Wynder, E. L. & Hoffmann, D. Tobacco and health: a societal challenge. N Engl J Med 300, 894–903 (1979).
Centers for Disease Control and Prevention (CDC). Quitting smoking among adults --- United States, 2001-2010. MMWR Morb Mortal Wkly Rep 60, 1513–1519 (2011).
Li, Q., Hsia, J. & Yang, G. Prevalence of smoking in China in 2010. N Engl J Med 364, 2469–2470 (2011).
Ojima, M., Hanioka, T., Tanaka, K., Inoshita, E. & Aoyama, H. Relationship between smoking status and periodontal conditions: findings from national databases in Japan. J Periodontal Res 41, 573–579 (2006).
Isaka, J. et al. Participation of periodontal ligament cells with regeneration of alveolar bone. J Periodontol 72, 314–323 (2001).
Pihlstrom, B. L., Michalowicz, B. S. & Johnson, N. W. Periodontal diseases. Lancet 366, 1809–1820 (2005).
Liu, Y. et al. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells 26, 1065–1073 (2008).
Ng, T. K., Lam, D. S. & Cheung, H. S. Prospects of stem cells for retinal diseases. Asia Pac J Ophthalmol 2, 57–63 (2013).
Park, D. et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 10, 259-272 (2012).
Huang, C. Y., Pelaez, D., Dominguez-Bendala, J., Garcia-Godoy, F. & Cheung, H. S. Plasticity of stem cells derived from adult periodontal ligament. Regen Med 4, 809–821 (2009).
Huang, L. et al. Directing adult human periodontal ligament-derived stem cells to retinal fate. Invest Ophthalmol Vis Sci 54, 3965–3974 (2013).
Fortino, V. R., Chen, R. S., Pelaez, D. & Cheung, H. S. Neurogenesis of neural crest-derived periodontal ligament stem cells by EGF and bFGF. J Cell Physiol 229, 479–488 (2014).
Morsczeck, C. et al. Somatic stem cells for regenerative dentistry. Clin Oral Investig 12, 113–118 (2008).
Krannitz, K. W., Fong, H. W., Fallat, L. M. & Kish, J. The effect of cigarette smoking on radiographic bone healing after elective foot surgery. J Foot Ankle Surg 48, 525–527 (2009).
Sørensen, L. T. Wound Healing and Infection in Surgery: The Clinical Impact of Smoking and Smoking Cessation: A Systematic Review and Meta-analysis. Arch Surg 147, 373–383 (2012).
Zhou, Y., Gan, Y. & Taylor, H. S. Cigarette smoke inhibits recruitment of bone-marrow-derived stem cells to the uterus. Reprod Toxicol 31, 123–127 (2011).
Chen, Y., Guo, Q., Pan, X., Qin, L. & Zhang, P. Smoking and impaired bone healing: will activation of cholinergic anti-inflammatory pathway be the bridge? Int Orthop 35, 1267–1270 (2011).
Kim, S. Y., Kang, K. L., Lee, J. C. & Heo, J. S. Nicotinic acetylcholine receptor α7 and β4 subunits contribute nicotine-induced apoptosis in periodontal ligament stem cells. Mol Cells 33, 343–350 (2012).
Ruiz, J. P., Pelaez, D., Dias, J., Ziebarth, N. M. & Cheung, H. S. The effect of nicotine on the mechanical properties of mesenchymal stem cells. Cell Health Cytoskelet 4, 29–35 (2012).
Ng, T. K. et al. Nicotine alters microRNA expression and hinders human adult stem cell regenerative potential. Stem Cells Dev 22, 781–790 (2013).
Boyland, E., Roe, F. J. & Gorrod, J. W. Induction of pulmonary tumours in mice by mitrosonornicotine, a possible constituent of tobacco smoke. Nature 202, 1126 (1964).
Tonetti, M. S. Cigarette smoking and periodontal diseases: etiology and management of disease. Ann Periodontol 3, 88–101 (1998).
Kaldahl, W. B., Johnson, G. K., Patil, K. D. & Kalkwarf, K. L. Levels of cigarette consumption and response to periodontal therapy. J Periodontol 67, 675–681 (1996).
Underner, M., Maes, I., Urban, T. & Meurice, J. C. Effects of smoking on periodontal disease. Rev Mal Respir 26, 1057–1073 (2009).
Zhou, Z. et al. Nicotine deteriorates the osteogenic differentiation of periodontal ligament stem cells through α7 nicotinic acetylcholine receptor regulating wnt pathway. PLoS One 8, e83102 (2013).
Frescaline, G., Bouderlique, T., Huynh, M. B., Papy-Garcia, D., Courty, J. & Albanese, P. Glycosaminoglycans mimetics potentiate the clonogenicity, proliferation, migration and differentiation properties of rat mesenchymal stem cells. Stem Cell Res 8, 180–192 (2012).
Ludwig, A. et al. Smoking decreases the level of circulating CD34+ progenitor cells in young healthy women--a pilot study. BMC Womens Health 10, 20 (2010).
Liu, X. D. et al. Cigarette smoke inhibits osteogenic differentiation and proliferation of human osteoprogenitor cells in monolayer and three-dimensional collagen gel culture. J Lab Clin Med 137, 208–219 (2001).
Gibon, E. et al. MC3T3-E1 Osteoprogenitor Cells Systemically Migrate to a Bone Defect and Enhance Bone Healing. Tissue Eng Part A 18, 968–973 (2012).
Liu, X. et al. Cigarette smoke extract inhibits chemotaxis and collagen gel contraction mediated by human bone marrow osteoprogenitor cells and osteoblast-like cells. Osteoporos Int 14, 235–242 (2003).
Giannopoulou, C., Geinoz, A. & Cimasoni, G. Effects of nicotine on periodontal ligament fibroblast in vitro. J Clin Periodontal 26, 49–55 (1999).
Han, E. K., Mcgonigal, T., Wang, J., Giranda, V. L. & Luo, Y. Functional analysis of focal adhesion kinase (FAK) reduction by small inhibitory RNAs. Anticancer Res 24, 3899–3905 (2004).
Li, H. & Fu, X. Mechanisms of action of mesenchymal stem cells in cutaneous wound repair and regeneration. Cell Tissue Res 348, 371–377 (2012).
Yoon, J. S., Lee, H. J., Chae, M. K., Lee, S. Y. & Lee, E. J. Cigarette smoke extract-induced adipogenesis in Graves' orbital fibroblasts is inhibited by quercetin via reduction in oxidative stress. J Endocrinol 216, 145–156 (2013).
Huang, J., Zhao, L., Xing, L. & Chen, D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells 28, 357–364 (2010).
Liu, X. et al. miR-18b inhibits TGF-β1-induced differentiation of hair follicle stem cells into smooth muscle cells by targeting SMAD2. Biochem Biophys Res Commun 438, 551–556 (2013).
Fonseca-Sanchéz, M. A. et al. microRNA-18b is upregulated in breast cancer and modulates genes involved in cell migration. Oncol Rep 30, 2399–2410 (2013).
Pelaez, D., Huang, C. Y. & Cheung, H. S. Isolation of pluripotent neural crest-derived stem cells from adult human tissues by connexin-43 enrichment. Stem Cells Dev 22, 2906–2914 (2013).
Martinez, C. et al. Periodontal ligament cells cultured under steady-flow environments demonstrate potential for use in heart valve tissue engineering. Tissue Eng Part A 19, 458–466 (2013).
Peran, M. et al. Activin/BMP2 chimeric ligands direct adipose-derived stem cells to chondrogenic differentiation. Stem Cell Res 10, 464–476 (2013).
Rui, Y. F., Lui, P. P., Wong, Y. M., Tan, Q. & Chan, K. M. BMP-2 stimulated non-tenogenic differentiation and promoted proteoglycan deposition of tendon-derived stem cells (TDSCs) in vitro. J Orthop Res 31, 746–753 (2013).
Dennis Jr, G. et al. DAVID: Database for Annotation, Visualization and Integrated Discovery. Genome Biol 4, P3 (2003).
Qiu, P. et al. Platelet-derived growth factor promotes the proliferation of human umbilical cord-derived mesenchymal stem cells. Cell Biochem Funct 31, 159–165 (2013).
Acknowledgements
We are grateful to the study subjects for their kind donation of their teeth. This work was supported in part by a block grant of the University Grants Committee Hong Kong and the Endowment Fund for Lim Por-Yen Eye Genetics Research Centre, Hong Kong and the VA Merit Review Grant and the Senior VA Research Career Scientist Award, Miami.
Author information
Authors and Affiliations
Contributions
T.K.N. and L.H. mainly performed the experiments. D.C. and Y.W.Y. helped with the experiments. W.M.T. recruited study subjects. C.P.P. and H.S.C. supervised the project. T.K.N., G.H.Y., C.P.P. and H.S.C. interpreted the results and wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Table 1
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Ng, T., Huang, L., Cao, D. et al. Cigarette smoking hinders human periodontal ligament-derived stem cell proliferation, migration and differentiation potentials. Sci Rep 5, 7828 (2015). https://doi.org/10.1038/srep07828
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07828
This article is cited by
-
Effects of smoking on the tissue regeneration-associated functions of human endometrial stem cells via a novel target gene SERPINB2
Stem Cell Research & Therapy (2022)
-
Attenuated regenerative properties in human periodontal ligament–derived stem cells of older donor ages with shorter telomere length and lower SSEA4 expression
Cell and Tissue Research (2020)
-
Pilot GWAS of caries in African-Americans shows genetic heterogeneity
BMC Oral Health (2019)
-
Disruption of retinal pigment epithelial cell properties under the exposure of cotinine
Scientific Reports (2017)
-
Expression and regulation of long noncoding RNAs during the osteogenic differentiation of periodontal ligament stem cells in the inflammatory microenvironment
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.