Abstract
Isoliquiritigenin (ILG) is a flavonoid derived from Glycyrrhiza uralensis and potently suppresses NLRP3 inflammasome activation resulting in the improvement of diet-induced adipose tissue inflammation. However, whether ILG affects other pathways besides the inflammasome in adipose tissue inflammation is unknown. We here show that ILG suppresses adipose tissue inflammation by affecting the paracrine loop containing saturated fatty acids and TNF-α by using a co-culture composed of adipocytes and macrophages. ILG suppressed inflammatory changes induced by the co-culture through inhibition of NF-κB activation. This effect was independent of either inhibition of inflammasome activation or activation of peroxisome proliferator-activated receptor-γ. Moreover, ILG suppressed TNF-α-induced activation of adipocytes, coincident with inhibition of IκBα phosphorylation. Additionally, TNF-α-mediated inhibition of Akt phosphorylation under insulin signaling was alleviated by ILG in adipocytes. ILG suppressed palmitic acid-induced activation of macrophages, with decreasing the level of phosphorylated Jnk expression. Intriguingly, ILG improved high fat diet-induced fibrosis in adipose tissue in vivo. Finally, ILG inhibited TLR4- or Mincle-stimulated expression of fibrosis-related genes in stromal vascular fraction from obese adipose tissue and macrophages in vitro. Thus, ILG can suppress adipose tissue inflammation by both inflammasome-dependent and -independent manners and attenuate adipose tissue fibrosis by targeting innate immune sensors.
Similar content being viewed by others
Introduction
Accumulating evidence suggests that obesity is associated with a state of chronic low-grade inflammation with infiltration of macrophages into adipose tissue, leading to insulin resistance1,2. Adipose tissue inflammation is increased by adipocyte lipolysis and adipose tissue fibrosis in obesity3. One study used a co-culture of adipocytes and macrophages to demonstrate a paracrine loop composed of saturated fatty acids (FAs) and TNF-α derived from adipocytes and macrophages, respectively4. Adipocyte derived endogenous FAs activate macrophages and induce TNF-α production, which in turn stimulates adipocytes and induces monocyte chemotactic protein-1 (MCP-1) expression in adipocytes, which is crucial for macrophage infiltration into adipose tissue5. Nuclear factor kappa light chain enhancer of activated B cells (NF-κB) and mitogen-activating protein (MAP) kinases, such as Erk (extracellular-signal-regulated kinase) and Jnk (c-Jun N-terminal kinase), are activated in the co-culture and play important roles in the induction of inflammatory changes in adipocytes and macrophages4,6.
The extracellular matrix is particularly important for maintaining the structural integrity of adipocytes and plays a critical role in adipogenesis. Fibrosis of adipose tissue may play an important role in adipose tissue dysfunction3. The connective fiber content of adipose tissue dramatically increases by the upregulation of collagen expression, which in turn elevates the overall rigidity of adipose tissue and finally leads to fibrosis. The deficiency of collagen 6, a key component of the extracellular matrix in adipose tissue, significantly improves the phenotypes of obese mice, including adipocyte death and adipose tissue inflammation3. This implies that alterations in the extracellular matrix in adipose tissue are linked to the development of inflammation. Thus, not only inflammation but also fibrosis may be important targets for the treatment of obesity.
Pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) quickly recognize pathogenic agents7. The TLR4/myeloid differentiation protein (MD)-2 complex is essential for lipopolysaccharide (LPS) recognition8,9. MD-2 reacts with LPS directly and this reaction induces homodimerization of the TLR4/MD-2 complex10. PRRs also recognize endogenous ligands called danger-associated molecular patterns (DAMPs). In the obese state, TLR4/MD-2 may recognize saturated free FAs, such as palmitic acid, derived from hypertrophied adipocytes as a DAMP and promote adipose tissue inflammation and insulin resistance4,6,11. Inflammasomes are multimeric protein complexes that are crucial for caspase-1 and IL-1β production12. The nucleotide-binding domain, leucine-rich repeats containing family, pyrin domain-containing-3 (NLRP3) inflammasome senses obesity-associated FAs and contributes to obesity-induced inflammation and insulin resistance13,14. Moreover, IL-1β inhibits insulin signaling in the insulin-target organs, including adipose tissue, liver, and skeletal muscle, and also induces dysfunction and cell death of insulin-producing pancreatic β cells15. Macrophage inducible C-type lectin (Mincle) recognizes not only cord factor, a mycobacterial glycolipid, but also SAP130 released from dead cells16,17. Furthermore, Mincle is highly expressed in M1 macrophages in adipose tissue and involved in the induction of adipose tissue fibrosis and insulin resistance during obesity18,19.
Isoliquiritigenin (ILG) is a component of Glycyrrhiza uralensis (G. uralensis) and a flavonoid with a chalcone structure. Its biological activities include anti-allergic20, anti-angiogenesis21, and anti-tumor growth22. A recent paper showed that ILG promotes regulatory T cell differentiation23. We reported previously that ILG suppressed LPS-TLR4/MD-2 complex signaling at the receptor level, resulting in inhibition of NF-κB and MAP kinases activation24. Furthermore, we found that ILG potently inhibited the activation of the NLRP3 inflammasome25. Our in vivo study revealed that the NLRP3 inflammasome of white adipose tissue (WAT) was an important target of ILG for improving diet-induced adipose tissue inflammation and insulin resistance25. However, it remains unclear whether ILG affects other pathways besides the inflammasome in adipose tissue inflammation. Additionally, little is known regarding whether ILG impacts fibrogenesis in adipose tissue.
This study explored the effects of ILG on adipose tissue inflammation and fibrosis. We now report that ILG suppressed adipose tissue inflammation by affecting the paracrine loop of the co-culture composed of adipocytes and macrophages. Furthermore, ILG markedly improved high fat diet (HFD)-induced adipose tissue fibrosis in vivo. We will discuss how ILG attenuates the inflammatory paracrine loop and adipose tissue fibrosis through inhibition of innate immune responses.
Results
Inflammatory responses in the co-culture of adipocytes and macrophages were suppressed by ILG stimulation independent of PPARγ activity
To examine the effects of ILG on adipose tissue inflammation, we used a co-culture system composed of differentiated 3T3-L1 adipocytes and RAW264.7 macrophages4,26 (Fig. 1A). Compared with bone marrow-derived macrophages (BMMs), RAW264.7 had low levels of IL-1β mRNA expression and mature IL-1β production by LPS and LPS plus ATP stimulation, respectively (Supplementary Fig. 1A,B). Whereas IL-1β mRNA expression was marginally but not significantly increased in the co-cultured cells compared with cells in the control (Supplementary Fig. 1C), mature IL-1β was not significantly produced by the co-culture of differentiated 3T3-L1 and either RAW264.7 or BMMs (Supplementary Fig. 1D).
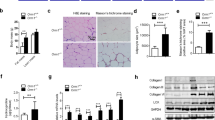
(A) Schematic diagram of the co-culture system. Differentiated 3T3-L1 adipocytes and RAW264.7 macrophages were co-cultured for 24 h. As a control culture, these cells were separately cultured and harvested for RT-qPCR. (B) RT-qPCR for TNF-α and MCP-1 mRNA in the control and co-cultured cells with or without pioglitazone (Pio) or ILG (n = 3 per group). Data are shown as means ± SD. N.S., not significant. #P < 0.01, §P < 0.001. (C) The interaction between CBP and PPARγ in the presence of pioglitazone (Pio) or ILG was determined as described in Methods. Data are shown as the mean ± SD. §P < 0.001. (D) 3T3-L1 cells were differentiated in the presence of pioglitazone (Pio) or ILG for 8 days as described in Methods. Representative Oil-Red-O staining images of 3T3-L1 cells are shown (n = 3 per group). The scale bars are 50 μM. (E) Spectrophotometric analysis of Oil-Red-O staining. Data are shown as means ± SD. N.S., not significant. §P < 0.001. All data are representative of at least three independent experiments.
We observed increased expression of TNF-α and MCP-1 mRNA in the co-cultured cells compared with the control culture (Fig. 1B). These expressions were decreased by ILG stimulation, albeit at high concentrations. As we previously reported26, a PPARγ agonist pioglitazone also decreased TNF-α and MCP-1 mRNA expression induced by the co-culture. We then investigated PPARγ agonistic activity of ILG by measuring the interaction between CREB binding protein (CBP) and PPARγ. ILG displayed much lower binding of PPARγ to CBP than pioglitazone, and the binding activity of 10 μM ILG was only one tenth that of 10 μM pioglitazone (Fig. 1C). To verify the low PPARγ agonistic activity of ILG, we examined the effect of ILG on adipogenesis. However, ILG had little effect on adipogenesis of differentiated 3T3-L1 cells (Fig. 1D,E). In parallel with the high PPARγ agonistic activity, pioglitazone significantly increased adipogenesis. Although several studies have shown that ILG inhibits cell growth and induces apoptosis27,28,29, the cell viabilities of RAW264.7 cells and 3T3-L1 adipocytes were not affected by ILG at the concentrations used (Supplementary Fig. 2). Taking these findings together, ILG suppresses inflammasome-independent inflammation in the co-culture of adipocytes and macrophages in vitro and PPARγ agonistic activity is not involved in the inhibitory effects of ILG.
ILG suppresses inflammatory changes elicited by the interaction between adipocytes and macrophages
To explore the mechanisms behind the inhibitory effects of ILG on the co-culture system, it was differently added to the culture of differentiated 3T3-L1 adipocytes and RAW264.7 macrophages as indicated in Supplementary Fig. 3. ILG stimulation decreased the levels of TNF-α and MCP-1 mRNA expression when it was added at the start of co-culture as shown in Fig. 1B (Fig. 2A, Group 1). In contrast, expressions of TNF-α and MCP-1 mRNA were not suppressed when ILG was added to either differentiated 3T3-L1 or RAW264.7 (Fig. 2A, Group 2 and Group 3). Those expressions were not affected when differentiated 3T3-L1 and RAW264.7 were treated with ILG individually before the co-culture (Fig. 2A, Group 4). These results indicate that ILG suppresses inflammatory changes elicited by the interaction between adipocytes and macrophages. Pioglitazone stimulation suppressed co-culture-induced TNF-α and MCP-1 mRNA expression when it was added at the start of co-culture as shown in Fig. 1B (Fig. 2B, Group 1). However, when RAW264.7 was treated with pioglitazone for 24 h before the co-culture, those expressions were not significantly affected (Fig. 2B, Group 2). In contrast, expressions of TNF-α and MCP-1 mRNA were decreased when pioglitazone was added to differentiated 3T3-L1 only or both differentiated 3T3-L1 and RAW264.7 before the co-culture (Fig. 2B, Group 3 and Group 4).
(A) Schematic diagram of the co-culture of differentiated 3T3-L1 adipocytes and RAW264.7 macrophages. (A,B) RT-qPCR for TNF-α and MCP-1 mRNA in the control and co-cultured cells with or without ILG (A) or pioglitazone (Pio) (B) (n = 3 per group). Data are shown as means ± SD. N.S., not significant. *P < 0.05, #P < 0.01, §P < 0.001. All data are representative of at least three independent experiments. (C) Differentiated 3T3-L1 adipocytes and RAW264.7 macrophages were co-cultured in the presence of pioglitazone (Pio) or ILG for 1 h. Phosphorylation (p) of IκBα, Jnk, and Erk was examined by western blotting. All data are representative of at least three independent experiments.
ILG attenuates NF-κB activation induced by the co-culture of adipocytes and macrophages
We then investigated the molecular mechanisms by which ILG suppressed inflammation in the co-culture. As NF-κB plays a critical role in the co-culture-induced inflammatory changes6, we firstly examined whether ILG affected the co-culture-induced NF-κB activation. We observed the phosphorylation of IκBα in the co-cultured cells compared with the control cells (Fig. 2C and Supplementary Fig. 4). This phosphorylation was potently inhibited by 3 and 10 μM of ILG. ILG stimulation also inhibited the co-culture-induced IκBα degradation (Fig. 2C). In contrast, the phosphorylation of IκBα was not obviously affected by pioglitazone stimulation (Fig. 2C and Supplementary Fig. 4). Activation of MAP kinases is also important for the induction of inflammatory changes of adipocytes and macrophages4. Phosphorylation of Erk was seen in the co-cultured cells compared with the control cells, whereas neither ILG nor pioglitazone stimulation inhibited this phosphorylation. Jnk and p38 MAP kinases were not phosphorylated by the co-culture compared with the control culture (Fig. 2C and data not shown).
ILG blunts TNF-α-induced insulin resistance in adipocytes through inhibition of NF-κB activation
TNF-α secreted by macrophages plays a critical role in the induction of inflammatory changes in adipocytes4. Stimulation of differentiated 3T3-L1 adipocytes with TNF-α induced MCP-1 mRNA expression and secretion (Fig. 3A,B). A high concentration of ILG (10 μM) significantly suppressed TNF-α-induced MCP-1 mRNA expression and secretion. Pioglitazone also potently decreased TNF-α-induced MCP-1 mRNA expression and secretion. Activation of NF-κB and MAP kinases plays critical roles in TNF-α-induced pro-inflammatory changes in adipocytes4,6. Phosphorylation and degradation of IκBα were observed in TNF-α-stimulated 3T3-L1 adipocytes (Fig. 3C and Supplementary Fig. 5A). ILG potently inhibited TNF-α-induced IκBα phosphorylation relative to pioglitazone. It was reported that NF-κB activation induced by TNF-α was not affected by pioglitazone stimulation30. Consistent with this report, TNF-α-induced IκBα phosphorylation was poorly inhibited by 10 μM of pioglitazone stimulation. Phosphorylation of Jnk and Erk was also observed in TNF-α-stimulated 3T3-L1 adipocytes (Fig. 3C). However, neither ILG nor pioglitazone had inhibitory effects on these changes.
(A,B) Differentiated 3T3-L1 adipocytes were treated with pioglitazone (Pio) or ILG for 6 h and subsequently stimulated with TNF-α (5 ng/ml) for 24 h (n = 3 per group). MCP-1 mRNA expression (A) and secretion (B) were measured by RT-qPCR and ELISA, respectively. Data are shown as means ± SD. N.S., not significant. #P < 0.01, §P < 0.001 (Medium vs. Vehicle, Vehicle vs. Pioglitazone or ILG). (C) Differentiated 3T3-L1 adipocytes were treated with pioglitazone (Pio) or ILG for 6 h and subsequently stimulated with TNF-α (1 ng/ml) for 10 min. Phosphorylation (p) of IκBα, Jnk, and Erk was examined by western blotting. (D) Differentiated 3T3-L1 adipocytes were treated with pioglitazone (Pio) or ILG for 6 h and subsequently stimulated with TNF-α (5 ng/ml) for 18 h. The cultured cells were treated with insulin for 30 min before ending of culture. Phosphorylation (p) of Akt was examined by western blotting. (E) Differentiated 3T3-L1 adipocytes were treated with ILG (10 μM) for 6 h and subsequently treated with indicated concentration of insulin for 30 min before ending of culture. Phosphorylation (p) of Akt was examined by western blotting. All data are representative of at least three independent experiments.
TNF-α stimulation induces insulin resistance through activation of NF-κB and MAP kinases in adipocytes31. Then, we examined whether ILG inhibits TNF-α-induced insulin resistance in adipocytes. TNF-α stimulation decreased insulin-induced Akt phosphorylation in differentiated 3T3-L1 adipocytes (Fig. 3D). ILG was effective in attenuating the reduction of Akt phosphorylation (Fig. 3D and Supplementary Fig. 5B). Although pioglitazone stimulation poorly affects TNF-α-induced NF-κB activation in adipocytes (Fig. 3C), Akt phosphorylation was restored by the stimulation (Fig. 3D and Supplementary Fig. 5B). We further examined whether ILG directly enhanced insulin signaling. Insulin-induced Akt phosphorylation was not augmented by even a high concentration of ILG (10 μM) (Fig. 3E and Supplementary Fig. 5C). Thus, ILG alleviates TNF-α-mediated insulin resistance in adipocytes, presumably through inhibition of NF-κB activation.
ILG but not pioglitazone suppresses palmitic acid-induced macrophage activation
Saturated FAs such as palmitic acid are important adipocyte-derived mediators of macrophage activation in co-culture4. Palmitic acid-induced macrophage activation was dependent on TLR4 signaling26. Furthermore, palmitic acid activates the NF-κB pathway through TLR46. Additionally, ILG attenuates lipid A, a biologically active component of LPS, -induced NF-κB activation24. Therefore, we explored the effects of ILG on palmitic acid-induced TLR4 activation. We firstly examined whether ILG affected FA release from adipocytes. TNF-α stimulation induced FA release from differentiated 3T3-L1 adipocytes in a dose-dependent manner (Supplementary Fig. 6). Whereas pioglitazone significantly decreased the FA release, ILG had no effect on this response. This suggests that PPARγ agonistic activity may be important for the inhibition of FA release from adipocytes. Then, we examined the effects of ILG on palmitic acid stimulation by using BMMs. BSA-conjugated palmitic acid (BSA-palmitic acid), but not BSA alone, significantly increased TNF-α mRNA and secretion in BMMs as previously reported (Fig.4A,B)26. ILG decreased BSA-palmitic acid-induced TNF-α mRNA expression in a dose-dependent manner (Fig. 4A). TNF-α secretion induced by BSA-palmitic acid was also significantly suppressed by 10 μM of ILG (Fig. 4B). In contrast, pioglitazone had no effect on these responses.
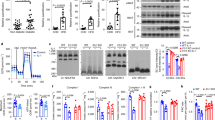
(A,B) BMMs were treated with pioglitazone (Pio) or ILG for 6 h and subsequently stimulated with BSA-palmitic acid (200 μM) for 24 h (n = 3 per group). TNF-α mRNA expression (A) and secretion (B) were measured by RT-qPCR and ELISA, respectively. Data are shown as means ± SD. N.S., not significant. *P < 0.05, §P < 0.001. (C, upper panel) Ba/F3 cells expressing murine TLR4/MD-2 and CD14 were treated with ILG (10 or 30 μM) for 1 h and subsequently stimulated with lipid A (3 ng/ml) for 18 h. The cells were harvested and GFP expression was monitored by flow cytometry. (Lower panel) Ba/F3 cells expressing murine TLR4/MD-2 and CD14 were stimulated with the indicated concentrations of BSA-palmitic acid for 18 h. The cells were harvested and GFP expression was monitored by flow cytometry. (D) BMMs were treated with pioglitazone (Pio) or ILG for 6 h and subsequently stimulated with BSA-palmitic acid (200 μM) for 2 h (n = 3 per group). Phosphorylation (p) of Jnk and Erk was examined by western blotting. All data are representative of at least three independent experiments.
Since the TLR4/NF-κB pathway plays a critical role in the saturated FA-induced inflammatory responses6, we examined the phosphorylation and degradation of IκBα in BSA-palmitic acid-stimulated BMMs by western blotting. However, IκBα phosphorylation and degradation in BMMs were not induced by even a high concentration of BSA-palmitic acid (100~400 μM) (data not shown). So we examined NF-κB activation by using Ba/F3 cells expressing murine TLR4/MD-2 complex and CD1424. The Ba/F3 cells were pretreated with ILG and then stimulated with lipid A or palmitic acid (Fig. 4C). NF-κB activation in the cultured cells was monitored by measuring GFP expression from a reporter construct using flow cytometry. Lipid A stimulation induced NF-κB activation and ILG pre-treatment inhibited its activation in a dose-dependent manner, as we previously reported (Fig. 4C, upper panel)24. In contrast, we did not observe NF-κB activation by BSA-palmitic acid stimulation (Fig. 4C, lower panel).
MAP kinases also play an important role in palmitic acid-induced TNF-α expression4. Phosphorylation of Jnk was observed in BSA-palmitic acid-stimulated BMMs (Fig. 4D). This phosphorylation was inhibited by ILG but not pioglitazone stimulation (Fig. 4D and Supplementary Fig. 7A). BSA-palmitic acid stimulation appeared to phosphorylate Erk, but its phosphorylation was also observed at BSA alone (Fig. 4D and Supplementary Fig. 7B). Phosphorylated p38 MAP kinase was not observed by BSA-palmitic acid stimulation (data not shown). These results indicate that ILG suppresses palmitic acid-induced TNF-α expression and production, presumably through inhibition of Jnk phosphorylation.
ILG attenuates LPS plus IFN-γ-induced differentiation to M1 macrophages
In vivo treatment of ILG to HFD-fed mice reduced the numbers of M1 and M2 macrophages in epididymal white adipose tissue (eWAT)25. Therefore, we examined the effects of ILG on the differentiation of M1 and M2 macrophages. 10 μM of ILG strongly suppressed the expression of M1 markers, including iNOS and TNF-α, in LPS plus IFN-γ-stimulated BMMs (Supplementary Fig. 8A). Pioglitazone treatment of HFD-fed mice decreased M1 markers and increased M2 markers in eWAT32. Consistent with this, pioglitazone suppressed the expression of M1 markers (Supplementary Fig. 8A). ILG had no effect on the expression of the M2 markers including arginase 1 and CD206 in IL-4-stimulated BMMs (Supplementary Fig. 8B). In contrast, pioglitazone significantly increased the expression of the M2 markers.
ILG supplementation improves HFD-induced adipose tissue fibrosis with decreased expression of TLR4 and Mincle
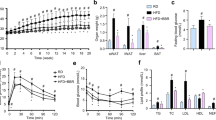
Adipose tissue exhibits interstitial fibrosis under the state of chronic inflammation or during the development of obesity3. Since ILG supplementation markedly improves HFD-induced adipose tissue inflammation and insulin resistance25, we examined whether ILG improves HFD-induced adipose tissue fibrosis. Male 5-week old C57BL/6 mice were fed a HFD, HFD supplemented with ILG (0.5% w/w; HFD-ILG) or normal diet (ND) for 20 weeks (Fig. 5A). Histological analysis clearly demonstrated that HFD induced extensive interstitial fibrosis in eWAT, which was markedly suppressed by ILG supplementation (Fig. 5B). HFD increased TNF-α and collagen 1 mRNA expression in eWAT (Fig. 5C). These expressions were markedly decreased by ILG supplementation, suggesting that ILG inhibits HFD-induced adipose tissue inflammation and fibrosis. HFD also increased TGF-β and TIMP-1 mRNA expression, which regulate extracellular matrix (ECM) production and ECM degradation, respectively. ILG supplementation markedly suppressed the increases of these expressions. Expression of PDGF-B, which regulates fibroblast proliferation, and TLR4 mRNA were marginally but not significantly increased by HFD. These increases were also marginally suppressed by ILG supplementation. Macrophage-inducible C-type lectin (Mincle) is an innate immune sensor that recognizes fungi and Mycobacterium tuberculosis16,33,34,35. Mincle is expressed selectively in adipose tissue macrophages18 and its expression was markedly up-regulated during obesity19. We confirmed that Mincle (Clec4e) mRNA expression in eWAT was up-regulated by HFD. Furthermore, ILG supplementation significantly decreased HFD-induced Mincle mRNA expression in eWAT. Additionally, the co-culture of differentiated 3T3-L1 adipocytes and RAW264.7 macrophages increased the levels of TLR4 and Mincle mRNA expression, which were suppressed by ILG stimulation in a dose-dependent manner (Supplementary Fig. 9).
(A) Schematic diagram of the experiment of ILG treatment to HFD-fed mice. (B) Representative images of Masson trichrome-stained sections of eWAT from mice fed with ND, HFD, or HFD-ILG for 20 weeks. Scale bars, 100 μm. Fibrotic area was analyzed by KEYENCE BZ-II Analyzer software. (C) RT-qPCR of TNF-α, collagen type 1, TGF-β, TIMP-1, PDGF-B, TLR4, and Mincle mRNA in eWAT from WT mice fed with ND, HFD or HFD-ILG for 20 weeks (n = 3 per group). Data are shown as the mean ± SE. N.S., not significant. *P < 0.05, #P < 0.01, §P < 0.001. Data are representative of at least two independent experiments.
ILG decreases TLR4- or Mincle-stimulated expression of fibrosis-related genes in SVF
TLR4 signaling in immune cells plays a key role in the development of obesity- and endotoxin-mediated adipose tissue fibrosis36. We examined whether ILG attenuated TLR4-stimulated expression of fibrosis-related genes in stromal vascular fraction (SVF) of obese eWAT (Fig. 6A). Lipid A stimulation for 24 h significantly increased TNF-α and Mincle mRNA expression in the SVF (Fig. 6B). Lipid A stimulation also increased the expression of TNF-α, Mincle, TGF-β, and TIMP-1 mRNA at 72 h stimulation. These increases were significantly attenuated by ILG stimulation. Expression of PDGF-B mRNA was not affected by lipid A stimulation or ILG stimulation.
(A) Schematic diagram of the stimulation of SVF. SVF from C57BL/6 mice fed with an HFD for 12 weeks was treated with vehicle or ILG for 6 h and subsequently stimulated with lipid A for 24 or 72 h. (B) RT-qPCR for TNF-α, Mincle, TGF-β, TIMP-1, and PDGF-B mRNA in the lipid A-stimulated SVF with or without ILG (n = 3 per group). Data are shown as the mean ± SE. *P < 0.05, #P < 0.01, §P < 0.001. Data are representative of at least two independent experiments.
Mincle stimulation is also crucial for fibrogenesis in SVF of obese adipose tissue18. The SVF from HFD-fed mice was stimulated with a Mincle ligand trehalose-6,6′-dimycolate (TDM), a mycobacterial cell wall glycolipid (Supplementary Fig. 10A). TDM stimulation for 24 h significantly increased TNF-α, Mincle, TGF-β, and PDGF-B mRNA expression in the SVF (Supplementary Fig. 10B). The effect of TDM was also observed at 72 h stimulation in the expression of TNF-α, Mincle, and TIMP-1. These increases were significantly attenuated by ILG stimulation. It was reported that mRNA expression of Acta2, which encodes the myofibroblast marker αSMA, was increased by TDM stimulation18. However, we did not confirm this increase of Acta2 (data not shown).
ILG decreases TLR4- or Mincle-stimulated fibrosis-related gene expression in macrophages, presumably through inhibition of NF-κB pathway
Finally, we explored the mechanisms behind the inhibitory effects of ILG on fibrogenesis. Activation of TLR4 and Mincle in monocytes or macrophages is crucial for the induction of fibrosis-related gene expression18,37. We examined whether ILG attenuated TLR4-stimulated their expression in macrophages (Fig. 7A). Lipid A stimulation for 24 or 72 h markedly increased TNF-α, TIMP-1, and PDGF-B mRNA expression in thioglycolate-elicited peritoneal macrophages (Fig. 7B). These increased expressions were significantly attenuated by ILG stimulation. Additionally, palmitic acid stimulation markedly increased Mincle mRNA expression in macrophages, which was significantly suppressed by ILG but not pioglitazone stimulation (Supplementary Fig. 11). Meanwhile, ILG had no effect on Mincle expression in lipid A-stimulated macrophages (Fig. 7B). Expression of TGF-β mRNA in macrophages was not increased by lipid A stimulation. A NF-κB inhibitor BAY11-7082 significantly suppressed lipid A-induced TNF-α and TIMP-1 mRNA expression at 24 and 72 h stimulation (Supplementary Fig. 12). In contrast, a MAP kinase inhibitor PD98059 exhibited weak suppression of TNF-α and TIMP-1 mRNA expression compared with BAY11-7082. ILG (30 μM) suppresses lipid A-induced phosphorylation of IκBα and MAP kinases in macrophages24. Western blotting analysis revealed that a lower concentration of ILG (3~10 μM) suppressed lipid A-induced phosphorylation of IκBα, Jnk, and p38 but not Erk (Supplementary Fig. 13 and Supplementary Fig. 14), which is effective in decreasing TNF-α and fibrosis-related gene expression (Fig. 7B).
(A) Schematic diagram of the stimulation of macrophages. Thioglycolate-elicited peritoneal macrophages from C57BL/6 mice were treated with vehicle or ILG for 6 h and subsequently stimulated with lipid A for 24 or 72 h. (B) RT-qPCR for TNF-α, Mincle, TGF-β, TIMP-1, and PDGF-B mRNA in the lipid A-stimulated macrophages with or without ILG (n = 3 per group). Data are shown as the mean ± SE. *P < 0.05, #P < 0.01, §P < 0.001. Data are representative of at least two independent experiments.
We further examined whether ILG attenuates Mincle-stimulated fibrosis-related gene expression in macrophages (Supplementary Fig. 15A). TDM stimulation for 24 h significantly increased TNF-α, Mincle, TIMP-1, and PDGF-B mRNA expression in thioglycolate-elicited peritoneal macrophages (Supplementary Fig. 15B). The effect of TDM was also observed at 72 h stimulation in the expression of TNF-α, Mincle, and TIMP-1 mRNA. These increases were significantly attenuated by ILG stimulation in a dose-dependent manner. Similar to lipid A stimulation, TGF-β mRNA expression was not increased by TDM stimulation. Additionally, BAY11-7082 potently suppressed TDM-induced TNF-α and TIMP-1 mRNA expression at 24 h stimulation relative to PD98059 (Supplementary Fig. 16).
Discussion
We previously identified ILG as a potent inhibitor of NLRP3 inflammasome25. ILG supplementation inhibits IL-1β and caspase-1 production in obese eWAT25. Since ILG has various biological activities, we investigated whether ILG inhibits adipose tissue inflammation in an inflammasome-independent manner. We used a co-culture system composed of adipocytes and macrophages, which induces inflammatory responses via an inflammasome-independent fashion. The ILG blocked the inflammatory paracrine loop containing TNF-α and saturated FA derived from macrophages and adipocytes, respectively, and consequently inhibited inflammatory responses induced by the co-culture via a PPARγ-independent fashion. (Fig. 8A). In adipocytes, ILG inhibited activation of the NF-κB pathway induced by TNF-α. As a result, ILG attenuated TNF-α-stimulated insulin resistance in adipocytes. Additionally, ILG inhibited palmitic acid-induced TNF-α expression and production in macrophages, with inhibition of MAP kinase activation. In contrast, pioglitazone did not have such inhibitory effects on macrophages. ILG improved diet-induced fibrosis in obese eWAT, and ILG inhibited the TLR4- and Mincle-stimulated fibrogenic program in SVF of obese eWAT and peritoneal macrophages (Fig. 8B). Our new findings revealed that ILG could attenuate adipose tissue inflammation in an inflammasome-independent fashion by targeting the interaction between adipocytes and macrophages. ILG can also attenuate adipose tissue fibrosis by inhibiting activation of innate immune sensors.
(A) ILG suppresses TNF-α-induced MCP-1 mRNA expression and secretion in adipocytes, coincident with inhibition of NF-κB activation. In addition, ILG restores inhibition of Akt phosphorylation under insulin receptor signaling in TNF-α-stimulated adipocytes. ILG also suppresses palmitic acid-induced TNF-α mRNA and secretion in macrophages, with decreasing the level of phosphorylated MAPK (Jnk) expression. It remains unclear whether NF-κB is involved in palmitic acid-induced TNF-α mRNA and secretion in macrophages. (B) Lipid A- or TDM-induced expression of fibrosis-related genes such as TIMP-1 is potently suppressed by a NF-κB inhibitor BAY11-7082 relative to a MAP kinase inhibitor PD98059, suggesting that NF-κB may play a key role in the induction of those expressions. ILG inhibits TLR4- or Mincle-stimulated expression of fibrosis-related genes in macrophages, presumably through inhibition of NF-κB pathway.
ILG acts on adipocytes and macrophages, and consequently suppresses inflammatory changes elicited by the co-culture (Fig. 2A). Furthermore, ILG inhibits TNF-α- and palmitic acid-induced activation of adipocytes and macrophages, respectively (Figs 3 and 4). Thus, ILG has inhibitory effects on two major cell types, which are involved in the induction of adipose tissue inflammation. In contrast, pioglitazone predominantly acts on adipocytes, thereby suppressing inflammatory responses elicited by the co-culture (Fig. 2B). Although PPARγ is highly expressed in activated macrophages and has an anti-inflammatory function38,39, pioglitazone did not suppress palmitic acid-induced macrophages activation (Fig.4A,B).
Murine 3T3-L1 preadipocytes are a valuable cell line and have been widely used to study adipocyte biology. However, they are immortalized cells and have distinctive attributes compared with primary human preadipocytes. For instance, 3T3-L1 cells become resistant to apoptosis induced by growth factor deprivation during their differentiation40,41. In contrast, human primary preadipocytes become more sensitive to apoptotic stimuli as they differentiate into mature adipocytes in vitro42. Therefore, results based on murine adipocyte cell lines may not be applicable for human adipocyte study. An in vitro system may have some limitations to examine the effects of ILG on human adipocytes.
NF-κB plays an important role in the induction of MCP-1 expression and TNF-α-induced insulin resistance in adipocytes6,43. PPAR agonists inhibit inflammatory responses at multiple steps of the NF-κB pathway in both PPAR-dependent and PPAR-independent manners44,45. PPARγ agonistic activity of ILG was much lower than that of pioglitazone (Fig. 1C–E), suggesting that ILG may inhibit NF-κB pathway in a PPARγ-independent manner. ILG interacts with IKK directly and inhibits its kinase activity in TNF-α-stimulated endothelial cells46. Therefore, IKK is a potential target for the inhibitory effect of ILG on TNF-α-stimulated adipocytes.
Our new findings are in line with the previous observation that ILG inhibits ligand-induced activation of TLR4/MD-2 in macrophages24. ILG inhibits activation of TLR4/MD-2 at the receptor level, namely LPS-induced its homodimerization24. However, homodimerization of TLR4/MD-2 did not occur by palmitic acid stimulation47. This suggests that inhibitory effects of ILG on palmitic acid stimulation may not be attributed to inhibition of TLR4/MD-2 homodimerization. As ILG inhibits palmitic acid-induced Jnk phosphorylation (Fig. 4D) or interacts with IKK directly46, it is possible that ILG is incorporated intracellularly and blocks TLR4 signaling at multiple steps. Future studies will determine a molecular target of ILG action on palmitic acid-induced TLR4 activation.
NF-κB inhibitors inhibit palmitic acid-induced TNF-α mRNA expression and secretion in macrophages6, suggesting that activation of NF-κB may play a critical role in FA-induced TLR4 activation. However, we and others have demonstrated that NF-κB activation was not induced by palmitic acid stimulation in the Ba/F3 cells stably expressing TLR4/MD-2 and CD14 and BMMs (Fig. 4C and data not shown)47. Although palmitic acid has been suggested to bind to MD-2, ligand-dependent homodimerization was not induced by palmitic acid stimulation47. These data indicate that palmitic acid may induce weak activation of NF-κB via TLR4/MD-2. Further investigation will reveal the impact of ILG on the TLR4-NF-κB pathway in macrophages.
Excessive collagen accumulation and the development of fibrosis can limit adipose tissue expandability, resulting in increased and ectopic lipid accumulation and the development of metabolic syndrome3. Here we demonstrated for the first time that ILG attenuates diet-induced adipose tissue fibrosis. The NLRP3 inflammasome activation of eWAT is an important target of ILG for improving adipose tissue inflammation25. Therefore, ILG has multiple targets in obese adipose tissue for preventing the development of metabolic syndrome. Moreover, ILG may prevent the development of adipose tissue fibrosis at an early stage of HFD feeding, consequently improving NLRP3 inflammasome-stimulated adipose tissue inflammation.
PRRs such as TLRs and C-type lectin receptors elicit inflammation and innate immune responses through activation of multiple signaling cascades. Mincle transduces activation signals by associating with the Fc receptor common γ-chain (FcRγ), which contains immunoreceptor tyrosine-based activation motifs (ITAMs) in the cytoplasmic domain. The phosphorylated ITAM recruits spleen tyrosine kinase (Syk), leading to activation of NF-κB and MAP kinases48,49. Given that an inhibitor against NF-κB or Syk suppresses TDM-stimulated TIMP-1 expression (Supplementary Fig. 16)18, the Syk-NF-κB pathway may be crucial for Mincle-stimulated fibrogenesis. Additional analysis will determine the precise mechanisms by which ILG inhibits the activation of Mincle signaling.
The expression level of Mincle is increased by various cellular stresses and stimuli. Mincle expression was upregulated in patients with rheumatoid arthritis50,51. A recent paper demonstrated that Mincle expression was increased in microglia, neuron, and endothelial cells in the brain after ischemic stroke51. Furthermore, Syk was phosphorylated in ischemic brain and a Syk inhibitor successfully prevented ischemic brain injury51. These results suggest that Mincle may be involved in the pathogenesis of ischemic stroke by initiating inflammatory responses. Therefore, Mincle may represent a potential target molecule for the treatment of inflammatory diseases, including rheumatoid arthritis, brain infarction, type 2 diabetes. Intriguingly, ILG decreased Mincle expression induced by various treatments including HFD, lipid A, palmitic acid, TDM and the co-culture (Figs 5C,6B and Supplementary Figs 9,10,11,15). Thus, ILG may be useful for treating various inflammatory diseases.
In conclusion, we here show that ILG suppresses an inflammatory paracrine loop between adipocytes and macrophages in an inflammasome-independent fashion and attenuates adipose tissue fibrosis through inhibition of a variety of innate immune responses. Additionally, besides macrophages, adipocytes are key cells for the inhibitory effects of ILG on adipose tissue inflammation and insulin resistance. Our results suggest that ILG could be a candidate for a therapeutic agent to treat both adipose tissue inflammation and fibrosis in obesity.
Methods
Mice
C57BL/6 mice were purchased from Japan SLC (Hamamatsu, Japan) and were used at 8 to 10 weeks of age. C57BL/6 mice were maintained in microisolator cages under specific pathogen-free conditions, and maintained in the animal facility of University of Toyama. Male 5-week old C57BL/6 mice were fed a HFD containing 60% fat (Research Diet, New Brunswick, NJ, USA), HFD supplemented with ILG (0.5% w/w) or ND containing 10% fat (Research Diet) for 20 weeks25. The experimental protocols were approved by the Animal Studies Committees at University of Toyama and Toyama Prefectural Institute for Pharmaceutical Research, and all experiments were performed according to the guidelines for the care and treatment of experimental animals at the institutes.
Reagents
ILG was purchased from AK Scientific Inc. (Union City, CA, USA) and Sigma-Aldrich (St. Louis, MO, USA). Pioglitazone was purchased from Funakoshi (Tokyo, Japan). ILG and pioglitazone were dissolved in dimethyl sulfoxide (DMSO). Recombinant mouse TNF-α, IL-4, and IFN-γ were purchased from R & D Systems (Minneapolis, MN, USA). Human insulin was purchased from Eli Lilly (Indianapolis, IN, USA). LPS from E. coli O55:B5, lipid A from Salmonella minnesota, palmitic acid, TDM, ATP, and thioglycolate were purchased from Sigma-Aldrich. Palmitic acid was conjugated to bovine serum albumin (BSA, Sigma-Aldrich) to increase solubility. BAY11-7082 was purchased from InvivoGen (San Diego, CA, USA). PD98059 was purchased from Merck Millipore (Darmstadt, Germany).
Cell culture
RAW264.7 cells (RIKEN BioResource Center, Tsukuba, Japan) and 3T3-L1 cells (kindly provided by Drs. Takayoshi Suganami and Yoshihiro Ogawa, Tokyo Medical and Dental University) were maintained with DMEM containing 10% FCS and antibiotics, and incubated at 37˚C in a humidified 5% CO2. 3T3-L1 cells were differentiated based on a standard protocol52. Accumulated lipid droplets were visualized using Oil-Red-O staining (Cosmo-Bio, Tokyo, Japan). For spectrophotometric analysis, Oil-Red-O was eluted with 100% isopropanol and measured at 540 nm with a microplate reader (Bio-Rad, Hercules, CA, USA). BMMs and BMMs-derived M1 and M2 macrophages were prepared as described previously26. Ba/F3 cells expressing murine TLR4/MD-2 and CD14 were kindly provided by Dr. Kensuke Miyake (The University of Tokyo).
PPARγ agonist activity
To measure PPARγ agonist activities of pioglitazone and ILG, the interaction between CBP and PPARγ was examined by using an ELISA kit (Fujikura Kasei, Tokyo, Japan) following the manufacture’s instructions.
Non-esterified fatty acid (NEFA) measurements
NEFA in the culture supernatants were measured with a colorimetric assay (Wako, Osaka, Japan) according to the manufacturer’s instruction.
Co-culture of adipocytes and macrophages
Co-culture of differentiated 3T3-L1 adipocytes and RAW264.7 macrophages was performed as described previously6. RAW264.7 macrophages were co-cultured with differentiated 3T3-L1 adipocytes in the absence or presence of pioglitazone or ILG for 24 h.
TDM stimulation
Thioglycolate-elicited peritoneal macrophages were obtained from C57BL/6 mice that had been injected intraperitoneally 4 days before with 3% thioglycolate solution. Isolation of SVF from eWAT of HFD-fed C57/BL6 mice was performed as described previously32. TDM dissolved in chloroform at 1 mg/ml were diluted in isopropanol and added on 12-well plate (2.5 μg of TDM/well), followed by evaporation of the solvent as described18. SVF (2.5×105/well) or peritoneal macrophages was cultured on the TDM coating plate in the absence or presence of ILG for 24 and 72 h.
ELISA
Culture supernatants were collected, and levels of MCP-1, TNF-α, and IL-1β in the culture supernatants were determined by using ELISA kits (R & D Systems).
Preparation of RNA and cDNA
Total RNA was isolated with RNeasy mini kit (QIAGEN, Hilden, Germany) and Sepasol® -RNA I Super G (Nacalai tesque, Kyoto, Japan) following the manufacture’s instructions. RNA was reverse transcribed with a TaqMan Reverse Transcription Reagents (Applied Biosystems, Carlsbad, CA, USA) following the manufacture’s instructions.
Real-time quantitative PCR
RT-qPCR was performed with a TaqMan Gene Expression Master Mix (Applied Biosystems) and analyzed with a CFX96 TouchTM Real-Time PCR Detection System (Bio-Rad) following the manufacture’s instructions. Relative transcript abundance was normalized for that of Hprt mRNA. The information for primers used for real-time PCR is listed in Supplementary Table 1.
Western blotting analysis
Cells were washed and lysed for 60 min in iced lysis buffer containing 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Triton X-100, protease inhibitor cocktail (Nacalai tesque). Lysates were subjected to SDS-PAGE and western blotting analyses. The following antibodies for immunoblotting were purchased from Cell Signaling (Beverly, MA, USA): anti-IκBα, anti-phospho-IκB, anti-phospho-Jnk, anti-Jnk, anti-phospho-Erk, anti-Erk, anti-phospho-p38, anti-p38, anti-phospho-Akt, anti-Akt. Anti-actin was purchased from Sigma-Aldrich. Goat anti-rabbit IgG alkaline phosphatase was purchased from Bio-Rad. Goat anti-mouse IgG alkaline phosphatase was purchased from American Qualex (San Clemonte, CA, USA). The reactive bands were visualized by ECL Plus (GE Healthcare, Uppsala, Sweden). Relative protein levels were quantified by using the ImageJ.
Cell viability assay
Cell viability assay was conducted by using a Cell Titer 96® Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA).
Histological analysis
Portions of the eWAT (n-2~3 per each group) were excised and fixed immediately with 4% formaldehyde at room temperature. Paraffin-embedded tissue sections were cut into 3-μm slices and placed on slides. Sections were stained with Masson trichrome stain. Twelve of eighteen images per each section were examined and fibrotic area was analyzed by KEYENCE BZ-II Analyzer software (KEYENCE, Osaka, Japan).
Statistical analysis
Statistical significance was evaluated by one-way ANOVA followed by post-hoc Tukey test.
Additional Information
How to cite this article: Watanabe, Y. et al. Isoliquiritigenin Attenuates Adipose Tissue Inflammation in vitro and Adipose Tissue Fibrosis through Inhibition of Innate Immune Responses in Mice. Sci. Rep. 6, 23097; doi: 10.1038/srep23097 (2016).
References
Hotamisligil, G. S. & Erbay, E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 8, 923–934, doi: 10.1038/nri2449 (2008).
Olefsky, J. M. & Glass, C. K. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72, 219–246, doi: 10.1146/annurev-physiol-021909-135846 (2010).
Khan, T. et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol 29, 1575–1591, doi: 10.1128/MCB.01300-08 (2009).
Suganami, T., Nishida, J. & Ogawa, Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arteriosclerosis, thrombosis, and vascular biology 25, 2062–2068, doi: 10.1161/01.ATV.0000183883.72263.13 (2005).
Kanda, H. et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116, 1494–1505, doi: 10.1172/JCI26498 (2006).
Suganami, T. et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arteriosclerosis, thrombosis, and vascular biology 27, 84–91, doi: 10.1161/01.ATV.0000251608.09329.9a (2007).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11, 373–384, doi: 10.1038/ni.1863 (2010).
Nagai, Y. et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol 3, 667–672, doi: 10.1038/ni809 (2002).
Shimazu, R. et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med 189, 1777–1782 (1999).
Akashi, S. et al. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J Exp Med 198, 1035–1042, doi: 10.1084/jem.20031076 (2003).
Shi, H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116, 3015–3025, doi: 10.1172/JCI28898 (2006).
Latz, E., Xiao, T. S. & Stutz, A. Activation and regulation of the inflammasomes. Nat Rev Immunol 13, 397–411, doi: 10.1038/nri3452 (2013).
Wen, H. et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 12, 408–415, doi: 10.1038/ni.2022 (2011).
Vandanmagsar, B. et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature medicine 17, 179–188, doi: 10.1038/nm.2279 (2011).
Tack, C. J., Stienstra, R., Joosten, L. A. & Netea, M. G. Inflammation links excess fat to insulin resistance: the role of the interleukin-1 family. Immunol Rev 249, 239–252, doi: 10.1111/j.1600-065X.2012.01145.x (2012).
Ishikawa, E. et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med 206, 2879–2888, doi: 10.1084/jem.20091750 (2009).
Yamasaki, S. et al. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol 9, 1179–1188, doi: 10.1038/ni.1651 (2008).
Tanaka, M. et al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat Commun 5, 4982, doi: 10.1038/ncomms5982 (2014).
Ichioka, M. et al. Increased expression of macrophage-inducible C-type lectin in adipose tissue of obese mice and humans. Diabetes 60, 819–826, doi: 10.2337/db10-0864 (2011).
Kakegawa, H., Matsumoto, H. & Satoh, T. Inhibitory effects of some natural products on the activation of hyaluronidase and their anti-allergic actions. Chem Pharm Bull (Tokyo) 40, 1439–1442 (1992).
Wang, Z. et al. Dietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via VEGF/VEGFR-2 signaling pathway. PLoS One 8, e68566, doi: 10.1371/journal.pone.0068566 (2013).
Lee, C. K. et al. Isoliquiritigenin inhibits tumor growth and protects the kidney and liver against chemotherapy-induced toxicity in a mouse xenograft model of colon carcinoma. J Pharmacol Sci 106, 444–451 (2008).
Guo, A., He, D., Xu, H. B., Geng, C. A. & Zhao, J. Promotion of regulatory T cell induction by immunomodulatory herbal medicine licorice and its two constituents. Sci Rep 5, 14046, doi: 10.1038/srep14046 (2015).
Honda, H. et al. Glycyrrhizin and isoliquiritigenin suppress the LPS sensor toll-like receptor 4/MD-2 complex signaling in a different manner. Journal of leukocyte biology 91, 967–976, doi: 10.1189/jlb.0112038 (2012).
Honda, H. et al. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. Journal of leukocyte biology 96, 1087–1100, doi: 10.1189/jlb.3A0114-005RR (2014).
Watanabe, Y. et al. The radioprotective 105/MD-1 complex contributes to diet-induced obesity and adipose tissue inflammation. Diabetes 61, 1199–1209, doi: 10.2337/db11-1182 (2012).
Iwashita, K., Kobori, M., Yamaki, K. & Tsushida, T. Flavonoids inhibit cell growth and induce apoptosis in B16 melanoma 4A5 cells. Biosci Biotechnol Biochem 64, 1813–1820, doi: 10.1271/bbb.64.1813 (2000).
Yadav, V. R., Prasad, S., Sung, B. & Aggarwal, B. B. The role of chalcones in suppression of NF-kappaB-mediated inflammation and cancer. Int Immunopharmacol 11, 295–309, doi: 10.1016/j.intimp.2010.12.006 (2011).
Chen, G. et al. Mammalian target of rapamycin regulates isoliquiritigenin-induced autophagic and apoptotic cell death in adenoid cystic carcinoma cells. Apoptosis 17, 90–101, doi: 10.1007/s10495-011-0658-1 (2012).
Peraldi, P., Xu, M. & Spiegelman, B. M. Thiazolidinediones block tumor necrosis factor-alpha-induced inhibition of insulin signaling. J Clin Invest 100, 1863–1869, doi: 10.1172/JCI119715 (1997).
Cheung, A. T. et al. An in vivo model for elucidation of the mechanism of tumor necrosis factor-alpha (TNF-alpha)-induced insulin resistance: evidence for differential regulation of insulin signaling by TNF-alpha. Endocrinology 139, 4928–4935, doi: 10.1210/endo.139.12.6336 (1998).
Fujisaka, S. et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 58, 2574–2582, doi: 10.2337/db08-1475 (2009).
Schoenen, H. et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol 184, 2756–2760, doi: 10.4049/jimmunol.0904013 (2010).
Wells, C. A. et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol 180, 7404–7413 (2008).
Yamasaki, S. et al. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci USA 106, 1897–1902, doi: 10.1073/pnas.0805177106 (2009).
Vila, I. K. et al. Immune cell Toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell Rep 7, 1116–1129, doi: 10.1016/j.celrep.2014.03.062 (2014).
Ciechomska, M. et al. Toll-like receptor-mediated, enhanced production of profibrotic TIMP-1 in monocytes from patients with systemic sclerosis: role of serum factors. Ann Rheum Dis 72, 1382–1389, doi: 10.1136/annrheumdis-2012-201958 (2013).
Ricote, M., Li, A. C., Willson, T. M., Kelly, C. J. & Glass, C. K. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391, 79–82, doi: 10.1038/34178 (1998).
Jiang, C., Ting, A. T. & Seed, B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 391, 82–86, doi: 10.1038/34184 (1998).
Magun, R., Boone, D. L., Tsang, B. K. & Sorisky, A. The effect of adipocyte differentiation on the capacity of 3T3-L1 cells to undergo apoptosis in response to growth factor deprivation. Int J Obes Relat Metab Disord 22, 567–571 (1998).
Magun, R., Gagnon, A., Yaraghi, Z. & Sorisky, A. Expression and regulation of neuronal apoptosis inhibitory protein during adipocyte differentiation. Diabetes 47, 1948–1952 (1998).
Papineau, D., Gagnon, A. & Sorisky, A. Apoptosis of human abdominal preadipocytes before and after differentiation into adipocytes in culture. Metabolism 52, 987–992, doi: 10.1016/s0026-0495(03)00165-3 (2003).
Ruan, H., Hacohen, N., Golub, T. R., Van Parijs, L. & Lodish, H. F. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes 51, 1319–1336 (2002).
Straus, D. S. et al. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A 97, 4844–4849 (2000).
Ruan, H., Pownall, H. J. & Lodish, H. F. Troglitazone antagonizes tumor necrosis factor-alpha-induced reprogramming of adipocyte gene expression by inhibiting the transcriptional regulatory functions of NF-kappaB. The Journal of biological chemistry 278, 28181–28192, doi: 10.1074/jbc.M303141200 (2003).
Kumar, S., Sharma, A., Madan, B., Singhal, V. & Ghosh, B. Isoliquiritigenin inhibits IkappaB kinase activity and ROS generation to block TNF-alpha induced expression of cell adhesion molecules on human endothelial cells. Biochem Pharmacol 73, 1602–1612, doi: 10.1016/j.bcp.2007.01.015 (2007).
Yamakawa, N. et al. Human TLR4 polymorphism D299G/T399I alters TLR4/MD-2 conformation and response to a weak ligand monophosphoryl lipid A. Int Immunol 25, 45–52, doi: 10.1093/intimm/dxs084 (2013).
Cargnello, M. & Roux, P. P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 75, 50–83, doi: 10.1128/MMBR.00031-10 (2011).
Vallabhapurapu, S. & Karin, M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 27, 693–733, doi: 10.1146/annurev.immunol.021908.132641 (2009).
Nakamura, N. et al. Isolation and expression profiling of genes upregulated in bone marrow-derived mononuclear cells of rheumatoid arthritis patients. DNA Res 13, 169–183, doi: 10.1093/dnares/dsl006 (2006).
Suzuki, Y. et al. Involvement of Mincle and Syk in the changes to innate immunity after ischemic stroke. Sci Rep 3, 3177, doi: 10.1038/srep03177 (2013).
Rubin, C. S., Hirsch, A., Fung, C. & Rosen, O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem 253, 7570–7578 (1978).
Acknowledgements
We thank all members of our laboratories in the University of Toyama and Toyama Prefectural Institute for Pharmaceutical Research for helpful discussions. We also thank Dr. Paul W. Kincade (Oklahoma Medical Research Foundation, Oklahoma City, OK, USA) for critical review of the manuscript. The authors sincerely thank Toyama Prefecture for supporting our laboratory. This work was supported by grants from Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) (JSPS KAKENHI Grant Numbers 25870257 to Y.W., 24590577 to Y.N., 24390119 to K.T., and 15K07960 to H.H.), JST, PRESTO (Y.N.), Hokuriku Innovation Cluster for Health Science, MEXT Regional Innovation Cluster Program, Toyama/Ishikawa Region (K.T.), Hokuriku Life Science Cluster, MEXT Regional Innovation Strategy Support Program (K.T.), Takeda Science Foundation (Y.W.), and First Bank of Toyama Scholarship Foundation (Y.W.).
Author information
Authors and Affiliations
Contributions
Y.N. conceived and designed the experiments. Y.W. conducted the experiments, collected data and analysed data. H.H. and N.O. conducted the experiments. S.Y., T.H., Y.I. and M.S. contributed the histological analysis. K.M. provided the Ba/F3 transfectant cell. M.T. and T.S. contributed Mincle-stimulated fibrosis data. Y.N. interpreted data and wrote the manuscript. K.T. was involved in project planning and supervision. Y.N. and K.T. are the senior authors. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Watanabe, Y., Nagai, Y., Honda, H. et al. Isoliquiritigenin Attenuates Adipose Tissue Inflammation in vitro and Adipose Tissue Fibrosis through Inhibition of Innate Immune Responses in Mice. Sci Rep 6, 23097 (2016). https://doi.org/10.1038/srep23097
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep23097
This article is cited by
-
LC-N2G: a local consistency approach for nutrigenomics data analysis
BMC Bioinformatics (2020)
-
Neuroprotective and anti-inflammatory effects of isoliquiritigenin in kainic acid-induced epileptic rats via the TLR4/MYD88 signaling pathway
Inflammopharmacology (2019)
-
Isoliquiritigenin suppresses human melanoma growth by targeting miR-301b/LRIG1 signaling
Journal of Experimental & Clinical Cancer Research (2018)
-
Isoliquiritigenin blunts osteoarthritis by inhibition of bone resorption and angiogenesis in subchondral bone
Scientific Reports (2018)
-
Aggressive Crosstalk Between Fatty Acids and Inflammation in Macrophages and Their Influence on Metabolic Homeostasis
Neurochemical Research (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.