Abstract
Bariatric surgery improves non-alcoholic fatty liver disease (NAFLD). Our aim was to investigate the potential role of ghrelin isoforms in the resolution of hepatic steatosis after sleeve gastrectomy, a restrictive bariatric surgery procedure, in diet-induced obese rats. Male Wistar rats (n = 161) were subjected to surgical (sham operation and sleeve gastrectomy) or dietary interventions [fed ad libitum a normal (ND) or a high-fat (HFD) diet or pair-fed]. Obese rats developed hepatosteatosis and showed decreased circulating desacyl ghrelin without changes in acylated ghrelin. Sleeve gastrectomy induced a dramatic decrease of desacyl ghrelin, but increased the acylated/desacyl ghrelin ratio. Moreover, sleeve gastrectomy reduced hepatic triglyceride content and lipogenic enzymes Mogat2 and Dgat1, increased mitochondrial DNA amount and induced AMPK-activated mitochondrial FFA β-oxidation and autophagy to a higher extent than caloric restriction. In primary rat hepatocytes, the incubation with both acylated and desacyl ghrelin (10, 100 and 1,000 pmol/L) significantly increased TG content, triggered AMPK-activated mitochondrial FFA β-oxidation and autophagy. Our data suggest that the decrease in the most abundant isoform, desacyl ghrelin, after sleeve gastrectomy contributes to the reduction of lipogenesis, whereas the increased relative acylated ghrelin levels activate factors involved in mitochondrial FFA β-oxidation and autophagy in obese rats, thereby ameliorating NAFLD.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a pathology characterized by intrahepatic triacylglycerol (TG) overaccumulation1,2, which is commonly associated with obesity, dyslipidemia, insulin resistance and type 2 diabetes3. NAFLD comprises a spectrum of liver disorders ranging from steatosis to non-alcoholic steatohepatitis (NASH) with risk of progression to liver cirrhosis and hepatocellular carcinoma4. The prevalence of NAFLD and NASH increases from around 20% and 3%, respectively, in the general population to 75% and 25–70%, respectively, in morbid obesity5,6. The excessive TG deposition in hepatocytes derives from an increased delivery of free fatty acids (FFA) into the liver from several different sources: excess dietary fat, higher FFA release from adipocytes via lipolysis, as well as increased de novo hepatic lipogenesis, ultimately leading to the development of obesity-associated NAFLD7. In addition, growing evidence indicates that hepatic mitochondrial dysfunction as well as alterations in autophagy are also involved in the development of this disease8. Bariatric surgery constitutes an effective treatment for morbid obesity achieving a more sustainable weight loss than that observed with lifestyle changes or pharmacological therapy9. Moreover, this procedure significantly improves, or even reverses, NAFLD, NASH and fibrosis in obese patients10,11,12. However, the molecular mechanisms underlying surgically-induced improvement of hepatic function remain unclear.
The orexigenic hormone ghrelin has been linked with the development of hepatosteatosis and the progression to NASH13. Ghrelin is a 28 amino acid peptide mainly synthesized in X/A cells of the oxyntic glands in the mucosa layer of the gastric fundus and circulates in two main forms: acylated ghrelin, with an n-octanoyl group at the serine 3 residue, and desacyl ghrelin, without this post-translational modification14,15,16. Administration of exogenous ghrelin increases food intake by activating hypothalamic neuropeptide Y/agouti-related peptide-containing neurons, which also express GHS-R type 1a, via the modulation of fatty acid metabolism17,18. Ghrelin also stimulates adiposity peripherally, through a direct stimulation of adipogenesis and lipogenesis in murine and human adipocytes19,20. The liver also constitutes a target for the lipogenic actions of ghrelin, since both chronic central and peripheral administration of ghrelin stimulate hepatic lipid storage21,22. In addition, opposite effects of ghrelin isoforms on hepatic glucose metabolism have been reported with desacyl ghrelin suppressing glucose release by hepatocytes as well as antagonizing the acylated ghrelin-induced increase in hepatic glucose output in vitro23. NAFLD is associated with alterations in total ghrelin concentrations24,25, but the specific role of acylated and desacyl in hepatic lipid metabolism has not been disentangled.
Circulating ghrelin concentrations decrease after sleeve gastrectomy due to the resection of the gastric fundus15 with RYGB additionally suppressing post-prandial ghrelin levels26. The aim of the present study was to analyze in diet-induced obese rats the implication of ghrelin isoforms in the improvement of hepatosteatosis after sleeve gastrectomy, a restrictive bariatric surgery procedure. In this regard, the potential differences in circulating acylated and desacyl ghrelin concentrations in obesity and after sleeve gastrectomy as well as their association with hepatic lipogenesis, lipophagy and mitochondrial FFA β-oxidation were evaluated. Moreover, the direct effects of the major isoforms of ghrelin on key regulatory molecules involved in these pathways were also studied in primary rat hepatocytes.
Results
Sleeve gastrectomy reduced serum desacyl ghrelin, but not the acylated form
Sleeve gastrectomy improved body weight, adiposity and insulin sensitivity of experimental animals (Tables 1 and 2). As expected, serum desacyl ghrelin was decreased in obese rats (P < 0.05) (Fig. 1A), but no significant changes in circulating acylated ghrelin and the acylated/desacyl ghrelin ratio were found between lean and obese rats (Fig. 1C and E). Rats undergoing bariatric surgery exhibited decreased (P < 0.05) desacyl ghrelin levels, without changes in acylated ghrelin concentrations (P = 0.820) (Fig. 1B and D). An increase in the acylated/desacyl ghrelin ratio (P < 0.05) was found after surgery (Fig. 1F).
Effect of sleeve gastrectomy on circulating ghrelin isoforms.
Bar graphs illustrate the impact of obesity and sleeve gastrectomy-induced weight loss on serum desacyl (A,B) and acylated (C,D) ghrelin levels as well as the acylated/desacyl ghrelin ratio (E,F). Differences were analyzed by Student’s t test or two-way ANOVA, where appropriate. *P < 0.05 vs lean control ND; bP < 0.05 effect of surgery.
Sleeve gastrectomy improved hepatic function and steatosis through the regulation of lipogenic factors
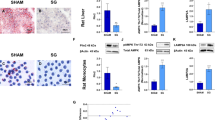
Obesity was associated with higher liver weight (P < 0.05), intrahepatic TG (P < 0.001) and serum AST and ALT levels, although the differences in transaminases did not read statistical significance (Table 1). Moreover, obese animals showed higher (P < 0.05) transcript levels of the lipogenic genes Pparg, Srebf1, Mogat2 and Dgat1 as well as a moderate-to-severe hepatic steatosis evidenced by the staining of the lipid droplet-coating protein adipophilin in liver sections (Fig. 2A–B and Supplemental Fig. 1). Liver weight (P < 0.01), intrahepatic TG (P < 0.05) and AST levels (P < 0.001) were decreased in rats subjected to bariatric surgery (Table 2). Accordingly, sleeve-gastrectomized rats exhibited a tendency towards a downregulation in hepatic Pparg (P = 0.084) and Srebf1 (P = 0.153) transcription factors as well as lower (P < 0.05) mRNA levels of Mogat2 and Dgat1 and mild steatosis evidenced by adipophilin staining (Fig. 2B–C and Supplemental Fig. 1).
Effect of acylated and desacyl ghrelin on the improvement of hepatic steatosis after sleeve gastrectomy.
Impact of obesity (A) and sleeve gastrectomy (C) on the mRNA expression levels of Pparg, Srebf1, Mogat2 and Dgat1 in liver samples of experimental animals. (B) Immunohistochemical detection of adipophilin in histological sections of rat liver (magnification 200x, scale bar = 100 μm). Effect of acylated (D,E) and desacyl (F,G) ghrelin on key lipogenic factors and intracellular triglycerides in rat hepatocyte cultures. The gene expression in lean rats, in the sham-operated groups fed a ND and in unstimulated hepatocytes was assumed to be 1. *P < 0.05; **P < 0.01 vs lean control ND or unstimulated hepatocytes; aP < 0.05 effect of diet; bP < 0.05 effect of surgery.
Acylated and desacyl ghrelin stimulate lipogenesis in primary rat hepatocytes
We next examined whether acylated and desacyl ghrelin levels are associated with the improvement in hepatic steatosis after sleeve gastrectomy. A positive correlation between desacyl ghrelin and ALT (r = 0.23, P < 0.05) was found, whereas acylated ghrelin was negatively correlated with intrahepatic TG (r = −0.36, P < 0.001). The direct effect of both ghrelin isoforms on lipogenesis was also evaluated in primary rat hepatocytes. Both acylated and desacyl ghrelin increased (P < 0.05) the mRNA expression of Mogat2 and Dgat1 and intracellular TG content in rat hepatocytes (Fig. 2D–F), although no significant changes were observed in Pparg and Srebf1 transcript levels.
Acylated and desacyl ghrelin induced changes in factors related to AMPK-induced hepatic mitochondrial β-oxidation
Since NAFLD is highly correlated with reduced mitochondrial lipid oxidation27, the effect of sleeve gastrectomy on the regulation of mitochondrial FFA β-oxidation pathway was evaluated. Obesity did not change the hepatic mitochondrial number, as evidenced by a similar mtDNA copy number and transcript levels of Tfam, a factor involved in mitochondrial genome replication (Fig. 3A–B). Interestingly, a significant (P < 0.05) increase in both markers of mitochondrial biogenesis was observed following sleeve gastrectomy (Fig. 3D–E). It is well established that activated AMPK directly phosphorylates and inactivates ACC, an enzyme that catalyzes the rate-limiting reaction for FFA synthesis28. The inhibition of ACC reduces the production of malonyl-CoA, an allosteric inhibitor of CPT1a, which is a key regulator of mitochondrial FFA uptake and its expression is positively regulated by PPARα29. Obese rats exhibited similar basal AMPK levels and AMPK phosphorylation/activation compared to lean rats (Fig. 3C). Accordingly, no differences (P > 0.05) in basal ACC levels, ACC phosphorylation/inactivation state as well as Ppara, CPT1A and FAS were observed in the obese group compared with lean rats (Fig. 3B–C). Interestingly, animals undergoing sleeve gastrectomy showed higher (P < 0.05) P-AMPK/AMPK and P-ACC/ACC ratios compared to the other interventional groups, as well as a marked upregulation (P < 0.05) of Ppara and Cpt1a expression, whereas CPT1A tended to increase (Fig. 3E–F).
Impact of sleeve gastrectomy on hepatic mitochondrial biogenesis and FFA β-oxidation.
Bar graphs show the effect of obesity (A,B) and sleeve gastrectomy (D,E) on the hepatic gene expression of molecules involved in mitochondrial biogenesis (mtDNA content and Tfam) and FFA β-oxidation (Ppara and Cpt1a). Representative cropped blots and Western-blot analysis show the impact of obesity (C) and sleeve gastrectomy (F) on the phosphorylation/activation of AMPK in Thr172 and the phosphorylation/inactivation of ACC in Ser79 as well as the basal expression of AMPK, ACC, FAS and CPT1A enzymes in liver samples of the experimental groups. The gene and protein expression in lean or in the sham group fed a ND was assumed to be 1. *P < 0.05 vs lean control ND; aP < 0.05 effect of diet; bP < 0.05 effect of surgery.
The ability of acylated and desacyl ghrelin to modulate mitochondrial FFA β-oxidation was also tested in rat hepatocytes. Both isoforms enhanced (P < 0.05) AMPK and ACC phosphorylation rates (Fig. 4C and F), without changing FAS and Tfam expression (Fig. 4A–F). Furthermore, the physiological concentration of acylated ghrelin significantly increased (P < 0.05) Ppara and Cpt1a transcripts (Fig. 4A), whereas the protein expression of CPT1A showed a similar trend only after acylated ghrelin stimulation, but differences did not reach statistical significance (Fig. 4C).
Effect of acylated and desacyl ghrelin on mitochondrial biogenesis and FFA β-oxidation.
Bar graphs show the effect of acylated (A) and desacyl (D) ghrelin on the expression of molecules involved in mitochondrial biogenesis (Tfam) and FFA β-oxidation (Ppara and Cpt1a). Representative cropped blots and Western-blot analysis showing impact of acylated (B,C) and desacyl (E,F) ghrelin on P-AMPK/AMPK and P-ACC/ACC ratios as well as the basal expression of AMPK, ACC, FAS and CPT1A enzymes in primary cultures of rat hepatocytes. The gene and protein expression in unstimulated hepatocytes was assumed to be 1. *P < 0.05; **P < 0.01 vs unstimulated hepatocytes.
Acylated ghrelin, and to a lesser extent desacyl ghrelin, activated hepatic autophagy
The role of autophagy, featured by an increased LC3B II/I ratio associated to a decreased p62/SQSTM1 protein content30, on the improvement of NAFLD after sleeve gastrectomy was next analysed. Obese rats showed similar hepatic expression of the autophagy-related genes Atg5 and Atg7 as well as in the LC3B-II/I ratio and the p62 protein level than lean rats (Fig. 5A–C). By contrast, sleeve gastrectomy was associated with an increase in Atg5 and Atg7 mRNA (Fig. 5D) and LC3B-II/I ratio together with a down-regulation of p62 (P < 0.05) (Fig. 5E–F). Interestingly, the acylated/desacyl ghrelin ratio was positively correlated with hepatic transcript levels of Atg5 (r = 0.22, P < 0.05). Thus, we next determined whether acylated and desacyl ghrelin directly affect autophagy in rat hepatocytes. The stimulation of hepatic cells with acylated ghrelin upregulated (P < 0.05) the expression of ATGs and LC3B-II/I ratio (Fig. 5G–H), while p62 levels were markedly diminished (Fig. 5I). However, desacyl ghrelin only modified the expression of p62 (P < 0.01) (Fig. 5L).
Impact of acylated and desacyl ghrelin on the activation of autophagy after sleeve gastrectomy.
Effect of obesity and sleeve gastrectomy on the expression of Atg5, Atg7 and Sqstm1 genes (A,D), autophagosome formation as evidenced by the LC3B-II/I ratio (B,E) as well as autophagy inhibition determined by p62 accumulation (C,F) in liver samples of the experimental groups. Representative cropped blots are shown at the top of the figures. Effect of acylated (G,H,I) and desacyl (J,K,L) ghrelin on key autophagy factors in rat hepatocyte cultures. The gene expression in lean rats, in the sham-operated groups fed a normal diet and in unstimulated hepatocytes was assumed to be 1. aP < 0.05 effect of diet; bP < 0.05 effect of surgery. *P < 0.05; **P < 0.01 vs unstimulated hepatocytes.
Discussion
Obesity is associated with an increased risk of NAFLD1,2 and surgically-induced weight loss improves serum transaminases and hepatic function31,32,33,34. In line with these observations, our data provide evidence that sleeve gastrectomy ameliorates hepatic function, as evidenced by an improved profile of AST and ALT, and hepatosteatosis, through the downregulation of lipogenic factors Pparg, Srebf1, Mogat2 and Dgat1. However, the molecular mechanisms underlying this amelioration remain undefined. Ghrelin has been recently proposed as a potential link between obesity and NAFLD35. In spite of its orexigenic and adipogenic properties, circulating total ghrelin concentrations are paradoxically decreased in obesity36. Our findings show that obese rats exhibited lower serum desacyl ghrelin levels without changes in acylated ghrelin and acylated/desacyl ghrelin ratio, which is in agreement with previous reports20,37,38,39. Interestingly, obese individuals with low ghrelin levels are more prone to weight regain after following a hypocaloric diet intervention40, suggesting an impaired central and/or peripheral ghrelin signalling that might explain the high-fat feeding in these subjects. One limitation of our study is that we only measured fasting ghrelin concentrations, and changes in post-feeding ghrelin levels are also important to understand their overall role in the regulation of appetite and metabolic flexibility. As expected, sleeve gastrectomy reduced desacyl ghrelin levels, due to the resection of the gastric fundus, the major production site of the hormone15. By contrast, plasma acylated ghrelin remained unchanged after bariatric surgery, which is in accordance with other authors26,41, and the acylated/desacyl ghrelin ratio increased, suggesting an enhanced post-transcriptional modification in order to maintain the circulating levels of the acylated hormone. In a previous work of our group, we found that both acylated and desacyl ghrelin stimulated intracellular lipid accumulation in human differentiated omental adipocytes through the upregulation of PPARγ and SREBP-1c and other fat-storage related molecules20. Accordingly, we herein show that both ghrelin isoforms also increase intracellular TG content in rat hepatocytes by increasing the transcript levels of the lipogenic factors Mogat2 and Dgat1. Since desacyl ghrelin represents ~90% of total ghrelin20,42, the amelioration of the hepatic function after sleeve gastrectomy might be partially due to the decrease of the desacylated hormone after the removal of the gastric fundus.
Sleeve gastrectomy was associated with an improvement in insulin sensitivity, as evidenced by lower insulinemia and HOMA, as well as lower glycemia, which is in accordance with previous results34. Opposite effects of acylated and desacyl ghrelin on insulin sensitivity have been reported. Although this might seem paradoxical, tight homeostatic control in some physiological processes is achieved by fine-tuning hormonal actions with opposite effects of the diverse isoforms43. Thus, exogenous desacyl ghrelin administration acts as a potent insulin secretagogue, whereas acylated ghrelin decreases glucose-induced insulin release and increases glucose levels in both humans and rodents44,45. In line with these observations, genetic, immunological, and pharmacological blockade of ghrelin signalling enhances glucose-stimulated insulin secretion and improves peripheral insulin sensitivity46. Thus, it seems plausible that ghrelin changes after sleeve gastrectomy also contribute, at least in part, to the observed improvement in insulin sensitivity after sleeve gastrectomy.
Obesity is associated with an increased release of FFA from adipocytes due to increased lipolysis7,47. Circulating FFA are taken up by the liver and metabolized by two main pathways: i) the mitochondrial β-oxidation to generate ATP; and ii) esterification to produce TG, which can be either incorporated into very-low density lipoprotein (VLDL) particles or stored within the hepatocytes48. AMPK coordinates the changes in the activity of enzymes of hepatic lipid metabolism through the regulation of the partitioning of FFA both in oxidative and biosynthetic pathways with defects in this pathway leading to the development of NAFLD49,50. In the present study, lack of changes in the basal AMPK and ACC expression and activity as well as in the mitochondrial copy number in obese rats might reflect an impaired AMPK transduction signalling and dysfunction in mitochondrial biogenesis in the rat steatotic liver, which is in accordance with previous studies of our group51 and others52,53. Interestingly, a notable increase in hepatic AMPK and ACC phosphorylation was found after sleeve gastrectomy, which is in agreement with other studies showing similar changes in AMPK and ACC activation after bariatric surgery54. Accordingly, an upregulation of hepatic Cpt1a and its upstream molecule Ppara as well as an increase in mitochondrial content was observed after sleeve gastrectomy, suggesting an increased flux of FFA towards mitochondrial β-oxidation and higher mitochondrial copy number rather than inducing de novo TG synthesis. Interestingly, ghrelin triggers AMPK signalling in rat ventricular cardiomyocytes, myoblasts, hypothalamic neurons and hepatocytes18,55,56,57. Analogously, we found that acylated and desacyl ghrelin promoted AMPK and ACC activation in primary rat hepatocytes, suggesting that this hormone may be involved in the molecular mechanisms whereby sleeve gastrectomy improves hepatic mitochondrial function. In addition, acylated ghrelin also upregulates Ppara and Cpt1a expression, while the mitochondrial content was not affected. This observation leads to the notion that acylated ghrelin should be considered an important effector improving mitochondrial β-oxidation efficiency, whereas the beneficial effects on mitochondrial biogenesis after the surgery are not mediated by this hormone.
Autophagy plays an important role in the regulation of hepatic lipid metabolism. In vitro and in vivo studies in murine hepatocytes and hepatic tissue have demonstrated that autophagy mediates the breakdown of lipid stores and that an inhibition of autophagy increases TG storage in lipid droplets leading to the development of a fatty liver8,58. The inhibition of autophagy sensitizes hepatocytes to palmitic acid-induced apoptosis, suggesting a pro-survival function of autophagy against lipotoxicity59. In this sense, decreased levels of autophagy have been found in the liver of severely obese leptin-deficient ob/ob mice as well as in obese, diabetic OLETF rats, which might promote lipid accumulation and impaired hepatic function in these animal models8,60. However, our results showed no changes in the hepatic gene expression of the autophagy-related factors Atg5 and Atg7, in the formation of autophagosomes evidenced by the LC3B-II/I conversion or in the inhibition of autophagy, expressed as p62 accumulation in obese rats. In this regard, hepatic autophagy is influenced by the degree of hepatic steatosis that might change the autophagosome formation during the ongoing NAFLD in adult obese rats. We herein report, for the first time, that sleeve gastrectomy is associated with an upregulation of the autophagy-related genes Atg5 and Atg7 and the LC3B-II/I ratio as well as with a decrease in the autophagy inhibition marker p62, suggesting that the induction of autophagy after this surgical procedure improves hepatic lipid clearance via lipophagy. Ghrelin isoforms are involved in the regulation of autophagy in several tissues, including the adipose tissue, small intestine, skeletal muscle and the heart38,61,62,63. Mao and colleagues reported that ghrelin induces autophagy in the liver via the activation of the AMPK signalling pathway56, but the specific role of each isoform of the hormone remains unclear. Interestingly, Mboat4-knockout mice lacking the gene encoding GOAT, which catalyzes the acylation of ghrelin, show a marked reduction of hepatic autophagy during fasting, suggesting an important role of acylated ghrelin in the regulation of autophagy in the liver during energy depletion35. In the present study, acylated ghrelin increased Atg5, Atg7 and the LC3B-II/I ratio and reduced p62 accumulation, while desacyl ghrelin only decreased p62 expression in hepatocytes, suggesting that acylated ghrelin and, to a lesser extent, desacyl ghrelin stimulate hepatic autophagy. Taken together, our findings suggest that the increase in acylated ghrelin levels after sleeve gastrectomy is involved in the induction of liver autophagy.
In summary, we herein show that the beneficial effects of bariatric surgery on NAFLD are mediated via a decrease in lipogenesis as well as an increase in autophagy and mitochondrial β-oxidation. The decrease in desacyl ghrelin after sleeve gastrectomy contributes to the reduction of lipogenesis, whereas the increased acylated ghrelin levels activate AMPK-activated mitochondrial FFA β-oxidation and autophagy in obese rats. These results support the notion that both ghrelin isoforms constitute key elements involved in the improvement of NAFLD after bariatric surgery.
Methods
Experimental animals and study design
Four-week-old male Wistar rats (n = 161) were fed ad libitum during 4 months with either a normal diet (ND) (n = 22) or a high-fat diet (HFD) (n = 139). Obese rats were randomized into weight-matched groups to be submitted either to the sleeve gastrectomy (n = 37) or a sham operation (n = 41). Anesthesia, sham surgery and sleeve gastrectomy were performed as earlier described64. Following the surgical interventions, a group of animals were fed ad libitum a HFD, while another group was switched to a ND. In order to discriminate the effects of a reduced food intake following the bariatric surgery, two groups of obese rats were pair-fed to the amount of food eaten by the animals undergoing the sleeve gastrectomy switched to either the ND (n = 17) or maintaining the HFD (n = 23). Four weeks after the surgical and dietary interventions, rats were killed by decapitation after an 8-h fast. The methods were carried out in accordance with the relevant guidelines and regulations. All experimental procedures were approved by the Ethical Committee for Animal Experimentation of the University of Navarra (049/10) and conformed to the European Guidelines for the Care and Use of Laboratory Animals (directive 2010/63/EU).
Blood and tissue analysis
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and intrahepatic TG were determined by enzymatic methods, as previously described65. Fasting acylated and desacyl ghrelin levels were also assessed using a rat/mouse EIA Kit (A05117 and A05118, Cayman Chemical, Ann Harbor, MI, USA).
RNA isolation and real-time PCR
RNA isolation and purification were performed as earlier described66. Transcript levels of Atg5, Atg7, Cav1, Cd36, Cpt1a, Dgat1, Fasn, Ghrl, Mboat4, Mogat2, Ppara, Pparg, Sqstm1, Srebf1 and Tfam (Supplementary Table S1) were quantified by real-time PCR (7300 Real Time PCR System, Applied Biosystems, Foster City, CA, USA). All results were normalized for the expression of 18 S rRNA (Applied Biosystems), and relative quantification was calculated as fold expression over the calibrator sample66.
Western-blot studies
Blots were incubated overnight at 4 °C with rabbit polyclonal anti-ACC, rabbit polyclonal anti-phospho-ACC (Ser79), rabbit polyclonal anti-AMPKα, rabbit monoclonal anti-phospho-AMPKα (Thr172), rabbit monoclonal anti-CPT1A, rabbit polyclonal anti-FAS, rabbit polyclonal anti-LC3B (Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit polyclonal anti-p62 (Sigma St. Louis, MO, USA) antibodies or murine monoclonal anti-β-actin (Sigma). The antigen-antibody complexes were visualized using horseradish peroxidase (HRP)-conjugated secondary antibodies and the ECL Plus detection system (Amersham Biosciences, Buckinghamshire, UK).
Immunohistochemistry of adipophilin
The immunodetection of the lipid droplet-marker adipophilin in liver sections was performed by the indirect immunoperoxidase method65, using a mouse monoclonal anti-adipophilin (Acris, Hiddenhausen, Germany) antibody. A semi-quantitative evaluation of steatosis in adipophilin-stained liver histological sections was performed. Hepatic steatosis was scored according to the staining of adipophilin by three blinded expert observers as: (0) none; (1) mild; (2) moderate; or (3) severe steatosis.
DNA extraction and analysis of mitochondrial DNA amount
The amount of mitochondrial DNA (mtDNA), extracted and purified using DNeasy Blood and Tissue Kit (Qiagen, Barcelona, Spain), was determined by real-time PCR of the mitochondrial cytochrome B (Cytb) gene normalized to the nuclear β-actin (Actb) gene (Supplementary Table S1), as previously described67. The real-time PCR was performed with 25 ng of total DNA using the TaqMan® Universal PCR Master Mix (Applied Biosystems), according to the manufacturer’s instructions.
Cell cultures
Primary rat hepatocytes were purchased from Tebu-Bio (Barcelona, Spain) and cultured in a collagen sandwich configuration68. Rat hepatocytes were seeded into 6-well plates (3 × 105 cells/well) and grown in Complete Growth Medium [DMEM/F-12 medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS), 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium (Invitrogen, Paisley, UK), 40 ng/mL dexamethasone (Sigma), 20 ng/mL epidermal growth factor (Sigma) and antibiotic-antimycotic] for 24 h. Then, cells were serum-starved for 24 h and stimulated with increasing concentrations (10, 100, 1000 pmol/L) of acylated and desacyl ghrelin, (Tocris, Ellisville, MO, USA) for 24 h. These physiological and supraphysiological concentrations of ghrelin isoforms to carry out the experiments were chosen on the basis of previous studies performed in our laboratory20,38.
Statistical analysis
Data are expressed as the mean ± SEM. Statistical differences between mean values were analyzed using Student’s t test, two-way ANOVA (diet × surgery), one-way ANOVA followed by Tukey’s or Dunnett’s post-hoc tests or the non-parametric Kruskal-Wallis test followed by U Mann-Whitney’s pairwise comparisons, where appropriate. Pearson’s correlation coefficients (r) were used to analyze the association between variables.
For more detailed Methods see Supplementary information.
Additional Information
How to cite this article: Ezquerro, S. et al. Acylated and desacyl ghrelin are associated with hepatic lipogenesis, β-oxidation and autophagy: role in NAFLD amelioration after sleeve gastrectomy in obese rats. Sci. Rep. 6, 39942; doi: 10.1038/srep39942 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Chalasani, N. et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 55, 2005–2023 (2012).
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) & European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 64, 1388–1402 (2016).
Utzschneider, K. M. & Kahn, S. E. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 91, 4753–4761 (2006).
Rinella, M. E. Nonalcoholic fatty liver disease: a systematic review. Jama. 313, 2263–2273 (2015).
Boza, C. et al. Predictors of nonalcoholic steatohepatitis (NASH) in obese patients undergoing gastric bypass. Obes Surg. 15, 1148–1153 (2005).
Machado, M., Marques-Vidal, P. & Cortez-Pinto, H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 45, 600–606 (2006).
Zhang, J. et al. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: a cross-sectional study. Sci Rep. 4, 5832 (2014).
Yang, L., Li, P., Fu, S., Calay, E. S. & Hotamisligil, G. S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 11, 467–478 (2010).
Frühbeck, G. Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nat Rev Endocrinol. 11, 465–477 (2015).
Lassailly, G., Caiazzo, R., Pattou, F. & Mathurin, P. Bariatric surgery for curing NASH in the morbidly obese? J Hepatol. 58, 1249–1251 (2013).
Taitano, A. A. et al. Bariatric surgery improves histological features of nonalcoholic fatty liver disease and liver fibrosis. J Gastrointest Surg. 19, 429–436, discussion 436–427 (2015).
Mathurin, P. et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 137, 532–540 (2009).
Zhang, S. R. & Fan, X. M. Ghrelin-ghrelin O-acyltransferase system in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 21, 3214–3222 (2015).
Hosoda, H., Kojima, M., Matsuo, H. & Kangawa, K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 279, 909–913 (2000).
Frühbeck, G., Díez Caballero, A. & Gil, M. J. Fundus functionality and ghrelin concentrations after bariatric surgery. N Engl J Med. 350, 308–309 (2004).
Rodríguez, A. Novel molecular aspects of ghrelin and leptin in the control of adipobiology and the cardiovascular system. Obes Facts. 7, 82–95 (2014).
Chen, H. Y. et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 145, 2607–2612 (2004).
López, M. et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 7, 389–399 (2008).
Gurriarán-Rodríguez, U. et al. Preproghrelin expression is a key target for insulin action on adipogenesis. J Endocrinol. 210, R1–7 (2011).
Rodríguez, A. et al. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes. 33, 541–552 (2009).
Sangiao-Alvarellos, S. et al. Central ghrelin regulates peripheral lipid metabolism in a growth hormone-independent fashion. Endocrinology. 150, 4562–4574 (2009).
Porteiro, B. et al. Ghrelin requires p53 to stimulate lipid storage in fat and liver. Endocrinology. 154, 3671–3679 (2013).
Gauna, C. et al. Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J Clin Endocrinol Metab. 90, 1055–1060 (2005).
Estep, M. et al. Association of obestatin, ghrelin, and inflammatory cytokines in obese patients with non-alcoholic fatty liver disease. Obes Surg. 21, 1750–1757 (2011).
Mykhalchyshyn, G., Kobyliak, N. & Bodnar, P. Diagnostic accuracy of acyl-ghrelin and it association with non-alcoholic fatty liver disease in type 2 diabetic patients. J Diabetes Metab Disord. 14, 44 (2015).
Malin, S. K. et al. Improved acylated ghrelin suppression at 2 years in obese patients with type 2 diabetes: effects of bariatric surgery vs standard medical therapy. Int J Obes. 38, 364–370 (2014).
Postic, C. & Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 118, 829–838 (2008).
Long, Y. C. & Zierath, J. R. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 116, 1776–1783 (2006).
Rakhshandehroo, M., Hooiveld, G., Muller, M. & Kersten, S. Comparative analysis of gene regulation by the transcription factor PPARalpha between mouse and human. PLoS One. 4, e6796 (2009).
Jansen, H. J. et al. Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology. 153, 5866–5874 (2012).
Wang, Y. & Liu, J. Sleeve gastrectomy relieves steatohepatitis in high-fat-diet-induced obese rats. Obes Surg. 19, 921–925 (2009).
Johansson, H. E., Haenni, A. & Zethelius, B. Platelet counts and liver enzymes after bariatric surgery. J Obes. 2013, 567984 (2013).
Myronovych, A. et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity. 22, 390–400 (2014).
Méndez-Giménez, L. et al. Sleeve gastrectomy reduces hepatic steatosis by improving the coordinated regulation of aquaglyceroporins in adipose tissue and liver in obese rats. Obes Surg. 25, 1723–1734 (2015).
Zhang, Y., Fang, F., Goldstein, J. L., Brown, M. S. & Zhao, T. J. Reduced autophagy in livers of fasted, fat-depleted, ghrelin-deficient mice: reversal by growth hormone. Proc Natl Acad Sci USA 112, 1226–1231 (2015).
Tschöp, M. et al. Circulating ghrelin levels are decreased in human obesity. Diabetes. 50, 707–709 (2001).
Rodríguez, A. et al. Association of plasma acylated ghrelin with blood pressure and left ventricular mass in patients with metabolic syndrome. J Hypertens. 28, 560–567 (2010).
Rodríguez, A. et al. The ghrelin O-acyltransferase-ghrelin system reduces TNF-alpha-induced apoptosis and autophagy in human visceral adipocytes. Diabetologia. 55, 3038–3050 (2012).
Barazzoni, R. et al. Gastric bypass does not normalize obesity-related changes in ghrelin profile and leads to higher acylated ghrelin fraction. Obesity. 21, 718–722 (2013).
Crujeiras, A. B. et al. Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J Clin Endocrinol Metab. 95, 5037–5044 (2010).
Patrikakos, P. et al. Long-term plasma ghrelin and leptin modulation after sleeve gastrectomy in Wistar rats in comparison with gastric tissue ghrelin expression. Obes Surg. 21, 1432–1437 (2011).
Pacifico, L. et al. Acylated and nonacylated ghrelin levels and their associations with insulin resistance in obese and normal weight children with metabolic syndrome. Eur J Endocrinol. 161, 861–870 (2009).
Frühbeck, G. & Gómez-Ambrosi, J. Control of body weight: a physiologic and transgenic perspective. Diabetologia. 46, 143–172 (2003).
Gauna, C. et al. Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. J Clin Endocrinol Metab. 89, 5035–5042 (2004).
Gauna, C. et al. Unacylated ghrelin acts as a potent insulin secretagogue in glucose-stimulated conditions. Am J Physiol Endocrinol Metab. 293, E697–E704 (2007).
Alamri, B. N., Shin, K., Chappe, V. & Anini, Y. The role of ghrelin in the regulation of glucose homeostasis. Horm Mol Biol Clin Investig. 26, 3–11 (2016).
Frühbeck, G., Méndez-Giménez, L., Fernández-Formoso, J. A., Fernández, S. & Rodríguez, A. Regulation of adipocyte lipolysis. Nutr Res Rev. 27, 63–93 (2014).
Browning, J. D. & Horton, J. D. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 114, 147–152 (2004).
Kahn, B. B., Alquier, T., Carling, D. & Hardie, D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 1, 15–25 (2005).
Yin, X., Li, Y., Xu, G., An, W. & Zhang, W. Ghrelin fluctuation, what determines its production? Acta Biochim Biophys Sin (Shanghai). 41, 188–197 (2009).
Rodríguez, A. et al. Impaired adiponectin-AMPK signalling in insulin-sensitive tissues of hypertensive rats. Life Sci. 83, 540–549 (2008).
Rector, R. S. et al. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 294, G619–626 (2008).
Finocchietto, P. V. et al. Defective leptin-AMP-dependent kinase pathway induces nitric oxide release and contributes to mitochondrial dysfunction and obesity in ob/ob mice. Antioxid Redox Signal. 15, 2395–2406 (2011).
Peng, Y. et al. Does LKB1 mediate activation of hepatic AMP-protein kinase (AMPK) and sirtuin1 (SIRT1) after Roux-en-Y gastric bypass in obese rats? J Gastrointest Surg. 14, 221–228 (2010).
Han, L. et al. Effects of ghrelin on triglyceride accumulation and glucose uptake in primary cultured rat myoblasts under palmitic acid-induced high fat conditions. Int J Endocrinol. 2015, 635863 (2015).
Mao, Y. et al. Ghrelin attenuated lipotoxicity via autophagy induction and nuclear factor-kB inhibition. Cell Physiol Biochem. 37, 563–576 (2015).
Yuan, M. J. et al. Ghrelin protects infarcted myocardium by induction of autophagy and AMP-activated protein kinase pathway. Biochem Biophys Res Commun. (2016).
Singh, R. et al. Autophagy regulates lipid metabolism. Nature. 458, 1131–1135 (2009).
Tan, S. H. et al. Induction of autophagy by palmitic acid via protein kinase C-mediated signaling pathway independent of mTOR (mammalian target of rapamycin). J Biol Chem. 287, 14364–14376 (2012).
Chang, E. et al. Ezetimibe improves hepatic steatosis in relation to autophagy in obese and diabetic rats. World J Gastroenterol. 21, 7754–7763 (2015).
Slupecka, M., Wolinski, J. & Pierzynowski, S. G. The effects of enteral ghrelin administration on the remodeling of the small intestinal mucosa in neonatal piglets. Regul Pept. 174, 38–45 (2012).
Yu, A. P. et al. Acylated and unacylated ghrelin inhibit doxorubicin-induced apoptosis in skeletal muscle. Acta Physiol. 211, 201–213 (2014).
Pei, X. M. et al. Protective effects of desacyl ghrelin on diabetic cardiomyopathy. Acta Diabetol. 52, 293–306 (2015).
Valentí, V. et al. Sleeve gastrectomy induces weight loss in diet-induced obese rats even if high-fat feeding is continued. Obes Surg. 21, 1438–1443 (2011).
Rodríguez, A. et al. Reduced hepatic aquaporin-9 and glycerol permeability are related to insulin resistance in non-alcoholic fatty liver disease. Int J Obes. 38, 1213–1220 (2014).
Catalán, V. et al. Validation of endogenous control genes in human adipose tissue: relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm Metab Res. 39, 495–500 (2007).
Heinonen, S. et al. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes. 64, 3135–3145 (2015).
Shulman, M. & Nahmias, Y. Long-term culture and coculture of primary rat and human hepatocytes. Methods Mol Biol. 945, 287–302 (2013).
Acknowledgements
The authors gratefully acknowledge Beatriz Ramírez (Metabolic Research Laboratory, Clínica Universidad de Navarra) for her technical assistance. SE was recipient of a predoctoral grant from the Asociación de Amigos of the University of Navarra. This work was supported by Fondo de Investigación Sanitaria-FEDER (PI12/00515 to GF and PI13/01430 to AR) from the Spanish Instituto de Salud Carlos III, the Department of Health of the Gobierno de Navarra (61/2014) to AR as well as by the Plan de Investigación de la Universidad de Navarra (PIUNA 2011-14) to AR.
Author information
Authors and Affiliations
Contributions
A.R. and G.F. designed the study. S.E., L.M.-G., S.B., R.M., V.V., V.C., J.G.-A., G.F. and A.R. researched the data. S.E., L.M.-G., S.B., V.C., J.G.-A., G.F. and A.R. contributed to the discussion. S.E., G.F. and A.R. wrote the manuscript. All authors reviewed/edited the manuscript and approved the final version. A.R. and G.F. are the guarantors of this work, had full access to all the data and take full responsibility for the integrity of data and the accuracy of data analysis.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ezquerro, S., Méndez-Giménez, L., Becerril, S. et al. Acylated and desacyl ghrelin are associated with hepatic lipogenesis, β-oxidation and autophagy: role in NAFLD amelioration after sleeve gastrectomy in obese rats. Sci Rep 6, 39942 (2016). https://doi.org/10.1038/srep39942
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep39942
This article is cited by
-
Molecular and cellular mechanisms underlying the hepatoprotective role of ghrelin against NAFLD progression
Journal of Physiology and Biochemistry (2023)
-
Role of ghrelin isoforms in the mitigation of hepatic inflammation, mitochondrial dysfunction, and endoplasmic reticulum stress after bariatric surgery in rats
International Journal of Obesity (2020)
-
Ghrelin and liver disease
Reviews in Endocrine and Metabolic Disorders (2020)
-
Electroacupuncture Attenuates Liver Inflammation in Nonalcoholic Fatty Liver Disease Rats
Inflammation (2020)
-
New insight into the mechanisms of ectopic fat deposition improvement after bariatric surgery
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.