Abstract
Metabolized by liver sinusoidal endothelial cells, autotaxin (ATX) is a secreted enzyme considered to be associated with liver damage. We sought to clarify the diagnostic ability of ATX for liver fibrosis in 593 biopsy-confirmed hepatitis C virus (HCV)-infected patients. The diagnostic accuracy of ATX was compared with clinical parameters and the established fibrosis biomarkers Wisteria floribunda agglutinin-positive Mac-2-binding protein, FIB-4 index, AST-to-platelet ratio, and Forn’s index. Median ATX levels were consistently higher in female controls and patients than in their male counterparts (P < 0.01). Serum ATX concentration increased significantly according to liver fibrosis stage in overall and both genders (P < 0.001). The cutoff values of ATX for prediction of fibrosis stages ≥F1, ≥F2, ≥F3, and F4 were 0.8, 1.1, 1.3, and 1.7 mg/L, respectively, in male patients and 0.9, 1.7, 1.8, and 2.0 mg/L, respectively, in female patients. The area under the receiver operating characteristic curve for ATX to diagnose fibrosis of ≥F2 (0.861) in male patients was superior to those of FIB-4 index and Forn’s index (P < 0.001), while that in female patients (0.801) was comparable with those of the other markers. ATX therefore represents a novel non-invasive biomarker for liver fibrosis in HCV-infected patients.
Similar content being viewed by others
Introduction
Persistent infection with HCV causes chronic hepatitis and eventually leads to liver cirrhosis and/or hepatocellular carcinoma (HCC)1. Although direct-acting antiviral agent (DAA) therapies have achieved high rates of a sustained virological response, chronic HCV infection remains a pressing health problem2,3,4,5,6. Disease progression to cirrhosis, hepatic failure, and complicating HCC, as well as esophageal and gastric varices, have been shown to positively correlate with histological involvement of the liver7. Thus, assessment of liver fibrosis stage in patients with chronic hepatitis C is deemed crucial in the decision of surveillance interval and therapeutic intervention8. Liver biopsy is the standard and most accurate examination method to evaluate liver fibrosis stage, but is invasive, prone to sampling error, and carries the risk of rare, but serious, complications9,10. Non-invasive biomarkers of disease progression are therefore needed. FIB-4 index11, aspartate aminotransferase (AST)-to-platelet ratio (APRI)12, and Forn’s index13 have all been established as non-invasive liver fibrosis markers and are useful in the clinical setting. Recently, Wisteria floribunda agglutinin-positive Mac-2-binding protein (WFA+-M2BP) was also discovered as a novel, noninvasive, serum glyco-marker for liver fibrosis in glycoproteomic biomarker screening studies14,15. This method has been validated for other liver diseases as well16,17.
Autotaxin (ATX) encoded by the ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (ENPP 2) gene is a secreted enzyme originally discovered in conditioned medium from A2058 human melanoma cell cultures18. ATX has important functions in converting lysophosphatidylcholine to lysophosphatidic acid (1- or 2-acyl-lysophosphatidic acid; LPA)19 involved in such physiological roles as cell migration, neurogenesis, angiogenesis, smooth-muscle contraction, platelet aggregation, and wound healing20,21. LPA also stimulates the proliferation and contractility of hepatic stellate cells. ATX was recently implicated in cancer metastasis and invasion as an autocrine motility factor22. Physiologically, the enzyme exists in the serum and is metabolized by liver sinusoidal endothelial cells. ATX metabolism is therefore considered to be reduced by liver fibrosis, leading to the elevation of serum values. These findings indicate that ATX may be directly related to liver fibrosis23,24,25. Although ATX levels reportedly vary significantly between genders in healthy subjects26, they nonetheless represent a novel serum marker of liver fibrosis27. However, the clinical features of ATX are inconclusive due to small cohort sizes.
This study aimed to clarify the clinical characteristics of ATX in patients chronically infected with HCV based on histological assessment evidence. We also compared the fibrosis predictive ability of ATX with that of other serum liver fibrosis markers.
Results
Baseline clinical characteristics
The baseline clinical characteristics of our cohort are summarized in Table 1. Of the 593 patients enrolled, 292 (49.2%) were male. Median age was 58 years. The vast majority of patients were seen for histological assessment prior to antiviral treatment with interferon-based therapy or DAAs. Fibrosis stage was F0 for 8 cases (1.3%), F1 for 284 cases (47.9%), F2 for 144 cases (24.3%), F3 for 103 cases (17.4%), and F4 for 54 cases (9.1%). Comparisons of baseline clinical characteristics between genders revealed several significant differences (Table 1), although the distributions of fibrosis stage and activity grade were comparable (P = 0.888 and P = 0.171, respectively).
ATX levels in controls and patients and between genders
Median ATX levels were significantly higher in patients than in healthy controls (1.40 vs. 0.76 mg/L; P < 0.001) (Fig. 1a). This relationship persisted when divided by gender (male: 1.16 vs. 0.70 mg/L; P < 0.001, female: 1.64 vs. 0.82 mg/L; P < 0.001) (Fig. 1b). In comparisons between genders, median ATX levels were significantly higher in women than in men both among healthy controls (0.82 vs. 0.70 mg/L; P < 0.001) as well as in patients with chronic hepatitis C (1.64 vs. 1.16 mg/L; P < 0.001) (Fig. 1b). Interestingly, FIB-4 index (P = 0.037) and Forn’s index (P = 0.011) were also higher in female patients, while WFA+-M2BP levels and APRI were comparable between genders (Table 1).
Correlation between ATX and liver fibrosis stage
We excluded the 8 patients (6 male and 2 female) who were diagnosed as having F0 stage from the analysis to evaluate the association between ATX and histologically significant fibrosis. Correlations between ATX and fibrosis stage (Fig. 2) and activity grade (Fig. 3) of the liver were investigated for all subjects and according to gender. Overall, median ATX in healthy controls and patients with fibrosis stage F1, F2, F3, and F4 was 0.76, 1.05, 1.64, 1.93, and 2.16, respectively. Median ATX levels in male healthy controls and male patients with fibrosis stage F1, F2, F3, and F4 were 0.70, 0.90, 1.33, 1.56, and 2.17 mg/L, respectively, while those in women were 0.82, 1.33, 1.96, 2.21, and 2.16 mg/L, respectively. Serum ATX increased significantly according to liver fibrosis progression (r = 0.72, P < 0.001 overall, r = 0.77, P < 0.001 in men, and r = 0.73, and P < 0.001 in women) and activity (r = 0.46, P < 0.001 overall, r = 0.69, P < 0.001 in men, and r = 0.66, P < 0.001 in women). Female ATX levels at each fibrosis stage were significantly higher than corresponding values in men (F1: P < 0.001, F2: P < 0.001, F3: P < 0.005) apart from F4 (P = 0.708).
Correlations between ATX and fibrosis markers
The correlation coefficients between ATX and other clinical markers (albumin, AST, alanine aminotransferase (ALT), γ-glutamyltransferase (GGT), alpha-fetoprotein (AFP), and platelet count) and non-invasive fibrosis markers (WFA+-M2BP, APRI, FIB-4 index, and Forn’s index) for male and female patients after analysis by Spearman’s rank correlation coefficient test are shown in Table 2. ATX was significantly positively correlated to AST, ALT, GGT, and AFP and significantly negatively correlated to albumin and platelet count. We observed the strongest correlation between ATX and WFA+-M2BP in total (r = 0.75, P < 0.001), male (r = 0.83, P < 0.001), and female (r = 0.71, P < 0.001) patients in addition to remarkable correlations with other fibrosis markers (Fig. 4).
Diagnostic performance of ATX
The calculated values of ATX for AUC, optimal cutoff value, sensitivity, specificity, positive predictive value, and negative predictive value for each fibrosis stage are listed in Table 3. The optimal cutoff values that best predicted fibrosis stage ≥F1 (vs. controls), ≥F2, ≥F3, and F4 were 0.9, 1.2, 1.5, and 1.7 mg/L, respectively, in all patients, 0.8, 1.1, 1.3, and 1.7 mg/L, respectively, in male patients, and 0.9, 1.7, 1.8, and 2.0 mg/L, respectively, in female patients.
Comparison of fibrosis markers by AUC
The ROC curves of ATX for predicting early fibrosis (≥F1 vs. controls), significant fibrosis (≥F2), severe fibrosis (≥F3), and cirrhosis (F4) in total, male, and female patients are presented in Fig. 5 and comparisons of AUC between ATX and other fibrosis markers in predicting fibrosis stage are summarized in Table 4. The AUC of ATX (0.901 overall, 0.910 in men, and 0.930 in women) for predicting early fibrosis (≥F1) was comparable to those of WFA+-M2BP and FIB-4 index. APRI was a significantly better predictor of early fibrosis than ATX for all subjects and both genders (0.982 [P < 0.01] overall, 0.985 [P < 0.01] in men, and 0.981 [P < 0.01] in women). The AUC of ATX (0.810 overall, 0.861 in men, and 0.801 in women) for predicting significant fibrosis (≥F2) was similar to those of WFA+-M2BP, FIB-4 index, APRI, and Forn’s index in women and superior to that of Forn’s index for all subjects (0.747 [P < 0.01]) and men (0.737 [P = 0.001]). The AUC of ATX (0.788 overall, 0.834 in men, and 0.782 in women) for prediction of severe fibrosis (≥F3) was comparable to those of WFA+-M2BP, FIB-4 index, APRI, and Forn’s index. For predicting cirrhosis, the AUC of ATX (0.796 overall, 0.862 in men, and 0.739 in women) closely reflected those of the other indicators.
Receiver operating characteristic curves for autotaxin (ATX) for the estimation of early fibrosis (≥F1), significant fibrosis (≥F2), severe fibrosis (≥F3), and cirrhosis (F4) in total (a), male (b), and female (c) patients. The number at the bottom center of each graph is the area under the curve. F, fibrosis.
Discussion
This investigation confirmed that ATX levels were significantly higher in women than in men in both healthy controls and patients with HCV, as earlier reported26,28. Accordingly, we compared ATX values with those of clinical markers according to gender in the study, and would advocate the same approach for assessing liver fibrosis stage with this biomarker. Tokumura et al. found serum ATX activity to be increased in normal pregnant women19, possibly since it also stabilizes blood vessels and is essential for blood vessel formation during fetal development29,30. However, the precise reason for this discrepancy remains under debate. ATX may participate in female reproductive biology, which is an important issue that requires investigation in future studies comparing ATX by gender.
Serum levels of ATX were verified to correlate both with liver fibrosis stage as well as with other clinical and non-invasive fibrosis markers in our study cohort of 593 patients that was substantially larger than a previous report of 74 patients27. Notably, ATX levels were validated by biopsy-determined histological scores in all patients. During the progression of liver fibrosis, liver sinusoidal endothelial cells are known to exhibit a loss of several receptors and sinusoidal endothelial fenestrae, causing the capillarization of sinusoids and uptake impairment of various substances31. ATX is generally considered to be high in the serum despite being cleared from the circulation and degraded in liver sinusoidal endothelial cells within minutes32. The phenotypic changes occurring in the liver during liver fibrosis may lead to reduced ATX clearance, thereby increasing circulating ATX concentration. Pleli et al. reported that ATX levels correlated closely with Child-Pugh stage and model of end stage liver disease (MELD) score, suggesting that ATX was an indicator of liver injury severity28. Although we could not address their findings since patients with hepatic failure were excluded from our cohort, our data confirmed that ATX level was highest in cirrhosis (F4).
ROC assessment of ATX confirmed the enzyme to be a comparably reliable marker for detecting fibrosis stage, especially F1 and F2. DAAs are currently the standard of care in chronic HCV infection as these IFN-free regimens achieve high rates of a sustained virological response with few side effects. Patients who are elderly or who exhibit persistently normal ALT levels are particularly well suited for DAA therapy. It is becoming difficult to perform liver biopsy in such individuals due to refusal and contraindications that include anticoagulant and antiplatelet agents. Moreover, most patients with normal ALT levels have none to early fibrosis33. ATX may therefore be useful to accurately determine early to significant fibrosis stage prior to initiating DAA treatment in these patients.
Interestingly, ATX was positively correlated with ALT and AFP as well as with histological activity grade. Elevated ALT and AFP reflect liver inflammation and regeneration. A recent study showed that ATX might be causally linked to immune activation during HCV infection and uncovered a decline in ATX to levels comparable to those of uninfected participants within 24 weeks of DAA therapy34. ATX was also correlated with transaminase and APRI, which was compatible with our own data. Hence, serum ATX level may be associated not only with liver fibrosis, but also with liver inflammation and regeneration.
A longitudinal investigation of patients with HCV with respect to ATX and clinical features, including long-term prognosis and liver carcinogenesis, is needed. The clinical utility of ATX should be assessed in other chronic liver diseases as well. In addition, the latest EASL NAFLD guidelines have established alcohol consumption of 20 g ethanol daily for females and 30 g daily for males to exclude alcoholic liver disease35,36. We had originally set this limit at 60 g ethanol/day in 1982 when the first liver biopsies were performed and therefore only had patient data for ≤ or >60 g/day. Such differences in alcohol intake may have influenced fibrosis differently in chronic hepatitis C and added bias to the results of this study. Lastly, we cannot exclude the possibility that ATX levels may have been underestimated in patients with liver cirrhosis since liver biopsy is sometimes contraindicated in such individuals in the clinical setting.
In conclusion, ATX represents an accurate, non-invasive biomarker for liver fibrosis estimation in patients with chronic HCV infection and merits more comprehensive establishment based on gender.
Methods
Subjects
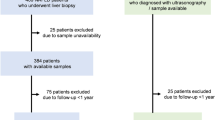
A total of 593 patients chronically infected with HCV, all of whom having received liver biopsy at Shinshu University Hospital in Matsumoto, Japan, between 1982 and 2015, were enrolled in this cross-sectional study. Their racial background was uniformly Japanese. The diagnosis of chronic hepatitis C was based on previously reported criteria as the presence of serum HCV antibodies and detectable HCV RNA37. One hundred and sixty subjects (80 male and 80 female) whose liver function tests were within normal levels were recruited as healthy controls. Age distribution was equally distributed among male and female controls (twenties: 20 subjects, thirties: 20 subjects, forties: 20 subjects, fifties: 20 subjects). All patients and controls were negative for hepatitis B surface antigen and antibodies to hepatitis B core antigen and the human immunodeficiency virus. No patients complicated with HCC were included. Patients who were diagnosed as having alcoholic liver disease, defined as an average daily consumption of >60 g of ethanol, were excluded. Patients who exhibited evidence of other liver disease, such as non-alcoholic liver disease, primary biliary cholangitis, or autoimmune hepatitis, were excluded as well. This study was reviewed and approved by the Institutional Review Board of Shinshu University Hospital (Matsumoto, Japan) (approval number: 3244), and written informed consent was obtained from all participating subjects. The investigation was conducted according to the principals of the Declaration of Helsinki.
Laboratory testing
All laboratory data were obtained on the same day or within 14 days after liver biopsy. ALT, AST, GGT, AFP, and other relevant biochemical tests were performed using standard methods.
Measurement of ATX
Blood samples were obtained on the same day or within 14 days after liver biopsy. Separated serum samples were immediately stored at −20 °C until testing.
Serum ATX antigen concentration was determined using a specific 2-site enzyme immunoassay. For preparation of the immunoassay, R10.23 was digested with pepsin, and the purified F(ab)2 form was isolated using phenyl-5PW hydrophobic column chromatography (Tosoh Co., Tokyo, Japan) to avoid the nonspecific binding of human antibodies to animal IgG types, such as human anti-mouse antibodies. In-house magnetic beads were coated with R10.23 F(ab)2 and placed in a reaction cup, and then 35 ng of alkaline phosphatase-labeled R10.21 in assay buffer (5% BSA, 5% sucrose, 10 mmol/l Tris–HCl, 10 mmol/l MgCl2, pH 7.4) was added. The ATX assay reagent was prepared by immediate freeze-drying of the reaction cup for subsequent use in a commercial automated immunoassay analyzer AIA-system (Tosoh Co.). The AIA-system process included automated specimen dispensation, incubation of the reaction cup, bound/free washing procedure, 4-methylumbelliferyl phosphate substrate dispensation, fluorometric detection, and result report. Antigen-antibody reaction time was 10 min and the first result was reported within 22 min. The throughput of the system was 60 samples/h using the AIA-600 II system26.
Liver biopsy and histological evaluation
Liver biopsies were performed on all patients by percutaneous sampling of the right lobe with a 14-gauge ACECUT needle (TSK LABORATORY Co., Hyogo, Japan) or by laparoscopic liver biopsy with a 13-gauge Silverman needle (Olympus Co., Tokyo, Japan). All biopsy specimens were 1.5 cm or more in length. Formalin-fixed, paraffin-embedded specimens were prepared and used for histopathological studies. Sections of 4 μm in thickness were cut from each paraffin block and stained with hematoxylin and eosin, periodic acid-Schiff after diastase digestion, or Azan-Mallory staining. All liver biopsy samples were independently evaluated by 2 investigators (AM and ET) who were blinded to the clinical data. Fibrosis stage and activity grade were assessed independently on each histological section by both investigators. In the case of a discrepancy, histological sections were simultaneously reviewed using a multi-pipe microscope to reach a consensus. Liver fibrosis stage (F0-4) and activity grade (A0-3) were determined according to the METAVIR scoring system38, as follows: F0 = no fibrosis, F1 = portal fibrosis without septa, F2 = portal fibrosis with few septa, F3 = numerous septa without cirrhosis, and F4 = cirrhosis. Significant fibrosis was defined as ≥F2, severe fibrosis as ≥F3, and cirrhosis as F4.
Other fibrosis markers
Serum WFA+-M2BP levels were measured simultaneously with ATX from stored serum samples obtained between 2006 and 2015. Serum WFA+-M2BP level was also measured in stored serum samples at the same point. Three additional surrogate blood indices of liver fibrosis were assessed at enrollment: FIB-4 index, APRI, and Forn’s index calculated according to the published formulae of (age [years] × AST [IU/L])/(platelet count [109/L] × ALT [IU/L]1/2)11, (AST/upper limit of normal; 40 IU/L) × (100/platelet count [109/L])12, and 7.811–3.131 × ln(platelet count [109/L]) + 0.781 × ln(GGT [IU/L]) + 3.467 × log(age [years]) − 0.014 × (cholesterol [mg/dL])13, respectively.
Statistical analysis
Statistical analysis and data visualization were carried out using IBM SPSS Statistics version 23.0 (IBM, Chicago, IL) and StatFlex version 6.0 (Artech Co., Ltd., Osaka, Japan) software. Data are presented as median ± interquartile range (IQR) for continuous variables. Groups were compared using the chi-square test for categorical variables. Correlations between fibrosis stage and serum ATX or other fibrosis markers were analyzed by means of Spearman’s rank test. Diagnostic accuracy was evaluated using the area under the receiver operating characteristic (ROC) curve (AUC). Comparisons of paired AUCs and 95% confidence intervals (CIs) were carried out using the nonparametric Delong test39. Cutoff values were identified by the Youden index, and the nearest clinically applicable value to the cutoff was considered as the optimal threshold for clinical convenience. All statistical tests were two-sided and evaluated at the 0.05 level of significance.
Additional Information
How to cite this article: Yamazaki, T. et al. Association of Serum Autotaxin Levels with Liver Fibrosis in Patients with Chronic Hepatitis C. Sci. Rep. 7, 46705; doi: 10.1038/srep46705 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Kiyosawa, K. et al. Interrelationship of blood transfusion, non-A, non-B hepatitis and hepatocellular carcinoma: analysis by detection of antibody to hepatitis C virus. Hepatology (Baltimore, Md.) 12, 671–675, doi: S0270913990002361 [pii] (1990).
Gane, E. J. et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med 368, 34–44, doi: 10.1056/NEJMoa1208953 (2013).
Poordad, F. et al. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med 368, 45–53, doi: 10.1056/NEJMoa1208809 (2013).
Kumada, H. et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology (Baltimore, Md.) 59, 2083–2091, doi: 10.1002/hep.27113 (2014).
Mizokami, M. et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. The Lancet. Infectious diseases 15, 645–653, doi: 10.1016/s1473-3099(15)70099-x (2015).
Kumada, H. et al. Randomized phase 3 trial of ombitasvir/paritaprevir/ritonavir for hepatitis C virus genotype 1b-infected Japanese patients with or without cirrhosis. Hepatology (Baltimore, Md.) 62, 1037–1046, doi: 10.1002/hep.27972 (2015).
Ge, P. S. & Runyon, B. A. Treatment of Patients with Cirrhosis. New England Journal of Medicine 375, 767–777, doi: 10.1056/NEJMra1504367 (2016).
Yano, M. et al. The long-term pathological evolution of chronic hepatitis C. Hepatology (Baltimore, Md.) 23, 1334–1340, doi: 10.1002/hep.510230607 (1996).
Bravo, A. A., Sheth, S. G. & Chopra, S. Liver biopsy. N Engl J Med 344, 495–500, doi: 10.1056/nejm200102153440706 (2001).
Cadranel, J. F., Rufat, P. & Degos, F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology (Baltimore, Md.) 32, 477–481, doi: 10.1053/jhep.2000.16602 (2000).
Sterling, R. K. et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology (Baltimore, Md.) 43, 1317–1325, doi: 10.1002/hep.21178 (2006).
Wai, C. T. et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology (Baltimore, Md.) 38, 518–526, doi: 10.1053/jhep.2003.50346 (2003).
Forns, X. et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology (Baltimore, Md.) 36, 986–992, doi: 10.1053/jhep.2002.36128 (2002).
Kuno, A. et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep 3, 1065, doi: 10.1038/srep01065 (2013).
Kuno, A. et al. Reconstruction of a robust glycodiagnostic agent supported by multiple lectin-assisted glycan profiling. Proteomics Clin Appl 7, 642–647, doi: 10.1002/prca.201300010 (2013).
Umemura, T. et al. Serum Wisteria floribunda Agglutinin-Positive Mac-2-Binding Protein Level Predicts Liver Fibrosis and Prognosis in Primary Biliary Cirrhosis. Am J Gastroenterol 110, 857–864, doi: 10.1038/ajg.2015.118 (2015).
Ichikawa, Y. et al. Serum Wisteria floribunda agglutinin-positive human Mac-2 binding protein may predict liver fibrosis and progression to hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Hepatol Resdoi: 10.1111/hepr.12712 (2016).
Stracke, M. L. et al. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem 267, 2524–2529 (1992).
Tokumura, A. et al. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem 277, 39436–39442, doi: 10.1074/jbc.M205623200 (2002).
Moolenaar, W. H. Lysophospholipids in the limelight: autotaxin takes center stage. J Cell Biol 158, 197–199, doi: 10.1083/jcb.200206094 (2002).
Moolenaar, W. H., van Meeteren, L. A. & Giepmans, B. N. The ins and outs of lysophosphatidic acid signaling. Bioessays 26, 870–881, doi: 10.1002/bies.20081 (2004).
Stracke, M. L., Clair, T. & Liotta, L. A. Autotaxin, tumor motility-stimulating exophosphodiesterase. Adv Enzyme Regul 37, 135–144 (1997).
Ikeda, H. et al. Effects of lysophosphatidic acid on proliferation of stellate cells and hepatocytes in culture. Biochem Biophys Res Commun 248, 436–440, doi: 10.1006/bbrc.1998.8983 (1998).
Yanase, M. et al. Lysophosphatidic acid enhances collagen gel contraction by hepatic stellate cells: association with rho-kinase. Biochem Biophys Res Commun 277, 72–78, doi: 10.1006/bbrc.2000.3634 (2000).
Yanase, M. et al. Functional diversity between Rho-kinase- and MLCK-mediated cytoskeletal actions in a myofibroblast-like hepatic stellate cell line. Biochem Biophys Res Commun 305, 223–228 (2003).
Nakamura, K. et al. Validation of an autotaxin enzyme immunoassay in human serum samples and its application to hypoalbuminemia differentiation. Clin Chim Acta 388, 51–58, doi: 10.1016/j.cca.2007.10.005 (2008).
Nakagawa, H. et al. Autotaxin as a novel serum marker of liver fibrosis. Clin Chim Acta 412, 1201–1206, doi: 10.1016/j.cca.2011.03.014 (2011).
Pleli, T. et al. Serum autotaxin is a parameter for the severity of liver cirrhosis and overall survival in patients with liver cirrhosis–a prospective cohort study. PloS one 9, e103532, doi: 10.1371/journal.pone.0103532 (2014).
Tanaka, M. et al. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem 281, 25822–25830, doi: 10.1074/jbc.M605142200 (2006).
van Meeteren, L. A. et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol 26, 5015–5022, doi: 10.1128/mcb.02419-05 (2006).
Muro, H., Shirasawa, H., Kosugi, I. & Nakamura, S. Defect of Fc receptors and phenotypical changes in sinusoidal endothelial cells in human liver cirrhosis. Am J Pathol 143, 105–120 (1993).
Jansen, S. et al. Rapid clearance of the circulating metastatic factor autotaxin by the scavenger receptors of liver sinusoidal endothelial cells. Cancer Lett 284, 216–221, doi: 10.1016/j.canlet.2009.04.029 (2009).
Okanoue, T. et al. A follow-up study to determine the value of liver biopsy and need for antiviral therapy for hepatitis C virus carriers with persistently normal serum aminotransferase. Journal of hepatology 43, 599–605, doi: 10.1016/j.jhep.2005.04.008 (2005).
Kostadinova, L. et al. During HCV and HCV-HIV infection elevated plasma Autotaxin is associated with LPA and markers of immune activation that normalize during IFN-free HCV therapy. The Journal of infectious diseasesdoi: 10.1093/infdis/jiw372 (2016).
EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Journal of hepatology 64, 1388–1402, doi: 10.1016/j.jhep.2015.11.004 (2016).
Ratziu, V., Bellentani, S., Cortez-Pinto, H., Day, C. & Marchesini, G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. Journal of hepatology 53, 372–384, doi: 10.1016/j.jhep.2010.04.008 (2010).
Umemura, T. et al. Quantitative analysis of anti-hepatitis C virus antibody-secreting B cells in patients with chronic hepatitis C. Hepatology (Baltimore, Md.) 43, 91–99, doi: 10.1002/hep.20917 (2006).
Bedossa, P. & Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology (Baltimore, Md.) 24, 289–293, doi: 10.1002/hep.510240201 (1996).
DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845 (1988).
Acknowledgements
The authors thank Mr. Trevor Ralph for his English editorial assistance.
Author information
Authors and Affiliations
Contributions
T.Y., S.J. and T.U. conceived and designed the experiments. Y.U. performed the experiments. T.Y., S.J. and T.U. analyzed the data. T.Y., S.J., T.U., A.S., N.F., S.S., Y.I., M.K., A.M., K.I., and E.T. contributed reagents, materials, and analytic tools. T.Y., S.J. and T.U. wrote the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Kouji Igarashi is an employee of TOSOH Corporation. The other authors declare that they have no conflicts of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yamazaki, T., Joshita, S., Umemura, T. et al. Association of Serum Autotaxin Levels with Liver Fibrosis in Patients with Chronic Hepatitis C. Sci Rep 7, 46705 (2017). https://doi.org/10.1038/srep46705
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep46705
This article is cited by
-
Serum autotaxin is a prognostic indicator of liver-related events in patients with non-alcoholic fatty liver disease
Communications Medicine (2024)
-
Autotaxin is a potential predictive marker for the development of veno-occlusive disease/sinusoidal obstruction syndrome after allogeneic hematopoietic cell transplantation
Annals of Hematology (2024)
-
Circulating thrombospondin 2 levels reflect fibrosis severity and disease activity in HCV-infected patients
Scientific Reports (2022)
-
Cholestatic Itch: Our Current Understanding of Pathophysiology and Treatments
American Journal of Clinical Dermatology (2022)
-
Clinical Usefulness of Serum Autotaxin for Early Prediction of Relapse in Male Patients with Type 1 Autoimmune Pancreatitis
Digestive Diseases and Sciences (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.