Abstract
Impairment of hippocampal neurogenesis has been associated with the expression of depressive-like symptoms and some studies have suggested neurogenesis as a critical factor in the normalization of behavior by antidepressant (AD) drugs. This study provides robust evidence that ongoing neurogenesis is essential for the maintenance of behavioral homeostasis and that its pharmacological arrest precipitates symptoms commonly found in depressed patients. Further, the incorporation of newly born neurons and astrocytes into the preexisting hippocampal neurocircuitry is shown to be necessary for the spontaneous recovery from the adverse effects of stress and for long-term benefits of AD treatments.
Similar content being viewed by others
Introduction
Major depression is a highly prevalent disorder that imposes a significant social burden. While the pathophysiology of the disease is still poorly understood, growing evidence suggests that impaired neuroplasticity may be a key underlying mechanism. Surrogate markers of impaired neuroplasticity, such as reduced neuroproliferation and impoverished dendritic arborization in the hippocampus, have been implicated in the onset, progression and remission of depressive symptoms.1, 2, 3, 4 Several antidepressants (ADs) tested to date stimulate hippocampal neurogenesis,5, 6, 7 but it is unclear as to whether these pro-neurogenic effects are responsible for their mood-, emotional- and cognitive-improving actions. Recent studies indicate that ADs exert their short-term therapeutic effects by inducing remodeling of dendrites and synapses in mood-regulating limbic brain regions rather than by stimulating neurogenesis per se;8, 9, 10, 11 however, considering the longitudinal course of depression, the debate on whether the long-term benefits of AD treatments result from altered hippocampal neurogenesis and gliogenesis remains open.
In this study, we examined whether and how the neurogenic process, from cell birth to integration of newly born cells into the existing circuitry, influences the development of and remission from depressive-like symptoms. Astrogliogenesis was included in the present analysis in light of evidence that astroyctes have a role in preventing of the expression of depression-like behaviors in laboratory animals.12, 13, 14 A validated unpredictable chronic mild stress (uCMS) paradigm was implemented to induce core symptoms of depressive-like behavior in rats; during the last 2 weeks of the uCMS protocol, animals were administered imipramine or fluoxetine. To assess the role of hippocampal neurogenesis and gliogenesis in the long-term effects of ADs, cell proliferation was artificially blocked through the coadministration of methylazoxymethanol (MAM) with the ADs. After 1 month of recovery from the experimental procedures, the behavioral dimensions commonly affected on depression were assessed and correlated with hippocampal neuroplastic alterations.
Materials and Methods
Animals and treatments
Male Wistar rats (200–250 g, aged 2 months; Charles-River Laboratories, Barcelona, Spain) were maintained under standard laboratory conditions (12 h light: 12 h dark cycles, 22 °C, relative humidity of 55%, ad libitum access to food and water). Groups of rats (n=10–12 per group) were randomly assigned to the following eight experimental groups: non-stress control+saline or MAM; stress (uCMS)+saline or MAM; uCMS+fluoxetine or imipramine alone, or coadministered with MAM. All procedures were carried out in accordance with EU Directive 2010/63/EU and NIH guidelines on animal care and experimentation.
A validated uCMS protocol was applied for 6 weeks as previously described.8, 15 The ADs fluoxetine (10 mg kg−1; Kemprotec, Middlesborough, UK) and imipramine (10 mg kg−1; Sigma-Aldrich, St Louis, MO, USA) were administered intraperitoneally (1 ml kg−1); MAM (7 mg kg−1; MRIGlobal Chemical Carcinogen Repository, Kansas City, MO, USA) was administered subcutaneously (0.45 ml kg−1). All drugs were dissolved in dimethyl sulfoxide (5%) and saline (0.9%) and administered daily, during the 2 last weeks of the uCMS protocol. Pilot studies showed that MAM did not adversely influence general health parameters, such as locomotor activity (ambulation in an open field, swimming speed in a water maze) and fur quality.8, 16, 17 Here, we found no significant alterations induced by MAM in appetite drive (Supplementary Figure S1), in swimming performance in the water maze (Supplementary Figure S2), as well as in weight variation (Supplementary Figure S3). BrdU was injected intraperitoneally (50 mg kg−1) for 5 days at the cessation of uCMS in order to evaluate neurogenesis by immunocytochemistry (see below).
Subsequently, animals were allowed to recover for 4 weeks and behavioral analyses were carried out at weeks 11 and 12, during the daily light phase (09:00–18:00 h).
Behavioral analysis
Sucrose consumption test
Anhedonia was assessed on a weekly basis by the sucrose consumption test, throughout the experimental procedures. Baseline sucrose preference values were established during a 1-week habituation period during which animals were presented with two pre-weighed drinking fluid bottles, containing water or 1% (m/v) sucrose. Before each recording of sucrose preference, rats were food- and water-deprived for 20 h and exposed to the test drinking solutions for 1 h. Sucrose preference was calculated as described previously.8
Elevated-plus maze
Anxiety-like behavior was examined through the elevated-plus maze (EPM) test, in a 5-min session, as previously described.15 The percentage of time spent in the open-arm was used as an index of anxiety-like behavior and the number of entries in the closed-arms was taken as an indicator for locomotor activity (Supplementary Figure S4).
Novelty suppressed feeding test
Anxiety-like traits were further assessed through the NSF paradigm. After a 18-h period of food-deprivation, animals were placed in an open-field arena, as previously described,8 where a single food pellet was positioned in the center. After reaching the pellet, animals were individually returned to their home cage, where pre-weighted food was available, and were allowed to feed during 10 min. The latency to feed in the open-field arena was used as an anxiety-like behavior measurement, whereas the food consumption in the animal home cages provided a measure of appetite drive. No differences were observed in the appetite drive between the experimental groups that could lead to a misinterpretation of the results (Supplementary Figure S1).
Forced swimming test
Learned-helplessness was assessed through the forced swimming test. Assays were conducted 24 h after a 5-min pretest session, by placing the rats in transparent cylinders filled with water (25 °C; 50 cm of depth) during 5 min. Trials were video-recorded and the immobility time, as well as the latency to immobility were measured using an automated video tracking system (Viewpoint, Champagne au mont d’or, France). Learned-helplessness was considered as an increase in the immobility time. Results were complemented with the analysis of latency to immobility, as a second measure of learned-helplessness (Supplementary Figure 5).
Cognitive assessment
Cognitive function was evaluated in a spatial working memory task and in a behavioral flexibility task, performed in a black circular tank (170 cm diameter) filled with water (23 °C; 31 cm of depth) placed in dimly lit room with extrinsic clues. The water tank was divided in four quadrants by imaginary lines and a black platform (12 cm diameter; 30 cm high), invisible to rodents, was placed in one of the quadrants. Trials were video-captured by a video-tracking system (Viewpoint). Animals were habituated to the test room, during the 2 days preceding the tests, being kept in the room for 1 h in each day. These tests were conducted as it follows:
Working memory task
The working memory task was used to evaluate the cognitive domain that relies on the interplay between the hippocampal and prefrontal cortex functions.18 The goal of this task, a modification of the original spatial reference memory test, is to assess the ability of animals to learn the position of the hidden platform and to retain this information during four consecutive trials. An escape platform was placed in one of the quadrants, and was maintained in the same position during the four daily trials. The test was performed during four days, and in each day the platform was repositioned in a new quadrant in a clockwise-fashion. In each of the daily trials animals were positioned in a different starting point (north, east, west and south) and a trial was considered as concluded when the platform was reached within the time-limit of 120 s. If the animals were unable to find the platform during the trial time they were guided to the platform and allowed to stay in it for 30 s. The time of escape latency was recorded for each trial.
Behavioral flexibility task
Following working memory assessment, tests were conducted for 4 days maintaining the platform in the same quadrant. At the fifth day, the behavioral flexibility performance of animals, a prefrontal cortex-dependent function, was tested by positioning the platform in a new (opposite) quadrant. Animals were tested in four trials according to the same procedure previously described. Besides the time of escape latency, the time spent in both new and old quadrants were recorded. Analysis of memory acquisition in the 4 days preceding was conducted in order to assess whether the different animal groups had equivalent memory for the old platform position (Supplementary Figure 6).
To confirm that differences observed in the escape latency were not due to distinct locomotor performance, we measured the average swimming velocities during trials. No differences were found among the groups in this parameter (Supplementary Figure S2).
Immunostaining procedures
Animals were deeply anaesthetized with sodium pentobarbital (20%; Eutasil, Sanofi) and were transcardially perfused with cold 4% paraformaldehyde. Brains were removed and post-fixed in 4% paraformaldehyde.
Coronal cryosections (20 μm) and vibratome sections (40 μm) were firstly stained for BrdU (1:50; Dako, Glostrup, Denmark), followed by staining for DCX (for neuroblasts, 1:500; Abcam, Cambridge, UK), NeuN (for mature neurons; 1:100; Chemicon, Temecula, CA, USA) or GFAP (for glia; 1:200; Dako). Finally, all sections were stained with 4',6-diamidino-2-phenylindole (1 μg ml−1). For each animal, BrdU-positive cells within the subgranular zone of the dentate gyrus were analyzed after double staining with neuronal (DCX or NeuN) or glial (GFAP) markers and cell counts were performed by confocal microscopy (Olympus FluoViewTM FV1000, Hamburg, Germany). Hippocampal proliferation at the end of the recovery period was assessed by Ki67 staining (1:200; Leica Microsystems, Wetzlar, Germany). Estimation of cell density in the dentate gyrus was obtained by crossing the cell number values with the corresponding dentate gyrus areas, determined using a Olympus BX51 optical microscope and the Newcast software (Visiopharm, Horsholm, Denmark).
Three-dimensional morphological analysis
To assess the three-dimensional dendritic morphology of characterized hippocampal neurons, we developed a new technique that combines Golgi-Cox impregnation with immunofluorescence staining. Briefly, brains were immersed in Golgi-Cox solution for 21 days and then transferred to a 30% sucrose solution and cut on a vibratome. Coronal sections (200 μm thick) were collected in 6% sucrose and blotted dry onto gelatin-coated microscope slides. They were subsequently alkalinized in 18.7% ammonia, developed in Dektol (Kodak, Rochester, NY, USA), fixed in Kodak Rapid Fix, dehydrated and xylene cleared. An adapted optimized BrdU immunostaining procedure was then applied in order to identify newly born neurons. Dendritic arborization, spine numbers and shape were, therefore, analyzed specifically in newborn BrdU+ cells and compared with non-BrdU+ neurons. The detailed method has been described in detail elsewhere.19
Statistical analysis
Statistical analyses were done using SPSS software (SPSS, Chicago, IL, USA). After confirmation of homogeneity, data was subjected to appropriate statistical tests. Analysis of variance repeated measures was used to analyze cognitive-learning tasks performance. One-way analysis of variance was used to evaluate the remaining behavioral and molecular results. F-values and P-values derived from the between groups analysis of variance analyses are properly indicated along the text. Differences between the groups were determined by Bonferroni’s post-hoc multiple comparison test, and the corresponding P-values are indicated in the figures. A t-test was used two evaluate differences among the two groups where appropriate. Statistical significance was accepted for P<0.05.
Results
Blockage of hippocampal proliferation triggers depressive-like symptomatology in naive rats
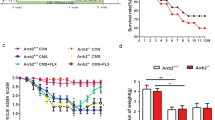
We first analyzed the long-term behavioral effects of neuro- and gliogenesis pharmacological suppression in naive animals (non-stressed animals), 4 weeks after the cessation of MAM treatment. Administration of MAM to naive rats, severely decreased the generation of neurons (BrdU+/NeuN+ cells, t8=6.024; P=0.0003) and astrocytes (BrdU+/GFAP+ cells, t8=2.889; P=0.020) (Figures 1a and b) and induced sustained deficits in hippocampal proliferation (Ki-67+ cells, t8=8.229; P<0.0001) (Figure 1c). As all neurons had matured 4 weeks after BrdU injections, we did not find DCX+/BrdU+ cells. Treatment with the antimitotic drug MAM produced increases in two surrogate measures of depressive-like behavior (reduced sucrose preference, a reflection of an anhedonic state, t18=1.941; P=0.034, Figure 1d; increased immobility in the FST, t18=3,889; P=0.001, Figure 1e). MAM administration also elicited signs of increased anxiety, as measured in the EPM (t18=4.069; P=0.0007, Figure 1f) and in the NSF (t18=4.324; P=0.0004, Figure 1g and Supplementary Figure S1), an interesting finding in light of the fact that a sizeable subpopulation of depressed human subjects exhibit hyperanxiety. In addition, MAM treatment was associated with impaired spatial working memory (F1,22=5.726; P=0.026, Figure 1h and Supplementary Figure S2) and behavioral flexibility (t18=4.158; P=0.0006, Figure 1i). Interestingly, new neurons (BrdU+ neurons), that escaped mitotic blockade, were found to have markedly reduced spine densities (t28=6.412; P<0.0001, Figure 1j) and altered spine morphology (Supplementary Figure S7), as compared with neurons that had matured before the experimental manipulations (Figure 1j).
Neurogenesis arrest induces long-term emotional and cognitive changes typical of depression. (a) Neurogenesis was arrested by methylazoxymethanol (MAM) administration and the effects on behavior were assessed after 4 weeks. MAM treatment decreased the number of BrdU-positive cells in the hippocampal dentate gyrus (b), that underwent neuronal (BrdU/NeuN) and astroglial (BrdU/GFAP) differentiation. (c) Deficits in proliferation were sustained 4 weeks after MAM treatment cessation. Behavioral phenotype was evaluated using a battery of tests to assess distinct behavioral domains affected in depression. (d, e) Long-term mood impairments were observed in the sucrose consumption test (SCT) (d), and in the forced swimming test (FST) (e) 4 weeks after MAM treatment. (f, g) Increased anxiety-like behavior was detected in the elevated plus maze test (EPM) (f) and in the novelty-suppressed feeding test paradigm (NSF) (g) in animals previously treated with MAM. (g, h) Cognitive performance was also affected 4 weeks after neurogenesis arrest, as both (h) working memory and (i) behavioral flexibility were impaired 4 weeks after MAM administration. MAM treatment did not affect the dendritic length of neither preexistent or newly born granule neurons (j), but there was a decrease in spine density in the dendrites of newly born neurons after MAM exposure. Error bars denote s.e.m. *P<0.05, **P<0.01, ***P<0.001; n=10–12 per group.
Hippocampal neurogenesis and gliogenesis are fundamental for sustained spontaneous and pharmacological recovery from depressive-like behavior
The importance of active neurogenesis in the precipitation of depressive-like behavior in animals exposed to uCMS, a validated animal model of depression,15, 20 was examined next. While most studies only report on immediate, possibly transient, recovery from stress, we here assessed ‘extended recovery’ by evaluating the display of depressive-like behavior 4 weeks after the cessation of stress (Figure 2a). In these experiments, MAM was administered during the last 2 weeks of AD treatment, allowing the examination of whether uninterrupted neurogenesis is necessary for long-term—spontaneous and AD treatment-associated—recovery from stress-induced depressive-like behavior. Like MAM, stress attenuated hippocampal neurogenesis and gliogenesis (F6,28=17.35, P<0.0001, post-hoc P<0.001 for neurons; F6,28=6.079; P=0.0004, post-hoc P<0.01 for glia; Figures 3a–d) and elicited signs of anhedonia in an AD-reversible manner. However, the AD actions occurred independently of ongoing neuroproliferation (Figures 2b and c). Animals exposed to uCMS only showed partial spontaneous recovery, as measured by the sucrose consumption test, but such behavioral recovery was absent in animals exposed to uCMS and MAM (F6,63=4.005; P=0.0019, post-hoc P<0.001, Figures 2b and c). The latter animals showed significantly reduced levels of neurogenesis (F6,28=26.80; P<0.0001, post-hoc P<0.001, Figure 3b) and proliferation (F6,28=26.80; P<0.0001, post-hoc P<0.001; Figures 3e and f) for up to 4 weeks after cessation of uCMS and MAM treatment. Strikingly, recovery during AD treatment was insensitive to the arrest of neurogenesis (Figures 2b and c). When tested in the forced swimming test (a test which measures reversal of learned helplessness within 24 h of AD treatment21), rats showed spontaneous and pharmacologically-induced recovery from the effects of uCMS, independently of ongoing neurogenesis (Figure 2d).
Neurogenesis arrest prevents long-term recovery from depression. (a) The relevance of neurogenesis for long-term recovery from depression was evaluated in animals exposed to an unpredictable chronic mild stress (uCMS) protocol 4 weeks before behavioral assessment. (b, c) Stress exposure triggers anhedonic behavior, that was reverted by fluoxetine and imipramine. Neurogenesis arrest with mehylazoxymethanol (MAM) in stressed animals precluded recovery from anhedonic signs in the sucrose consumption test (SCT). (d) Learned helplessness behavior, assessed in the forced swimming test (FST), was normalized after 4 weeks of spontaneous or antidepressant-induced recovery from stress. (e, f) The long-term recovery from anxiety-like behavior was prevented by neurogenesis arrest; fluoxetine anxiolytic effects were attenuated by MAM administration, while imipramine action remained unaffected. (g–j) Neurogenesis arrest prevented the recovery form cognitive deficits in (g) behavioral flexibility and in (h) working memory of animals exposed to uCMS; (i, j) the therapeutical action of fluoxetine on working memory was suppressed by MAM administration, while imipramine effect was maintained after neurogenesis arrest. Error bars denote s.e.m. *Denotes the effect of MAM; ⋄Denotes the effect of uCMS; #Denotes the antidepressants effect, by comparison of the antidepressants-treated animals with uCMS animals. *,⋄, #P<0.05, **,⋄⋄, ##P<0.01, ***,⋄⋄⋄, ###P<0.001; n=10–12 per group. EPM, elevated plus maze test; NSF, novelty-suppressed feeding test paradigm.
Pro-neurogenic and pro-gliogenic actions of the antidepressants fluoxetine and imipramine are associated with emotional and cognitive long-term recovery from depression. (a, b) Micrographs depict examples of (a) BrdU/NeuN double-labeled neurons and (b) BrdU/GFAP double-labeled glial cells in the hippocampal dentate-gyrus (DG) of control animals. (c, d) Graphs show the density of (c) total BrdU labeled cells and of (d) BrdU/NeuN, to evaluate neurogenesis, and BrdU/GFAP double-labeled cells, to evaluate gliogenesis, in the hippocampal DG. Unpredictable chronic mild stress (uCMS) animals, with or without methylazoxymethanol (MAM) treatment, have significantly lower levels of neurogenesis and gliogenesis when compared with control animals. Fluoxetine and imipramine treatment leads to a recovery in the levels of neurogenesis and gliogenesis, respectively. (e) Proliferation analyses, assessed at the end of the 4-weeks recovery period, by Ki67 staining, showed that uCMS and antidepressant-treated animals have similar proliferation levels to control animals. The uCMS effect on proliferation was prevented by neurogenesis arrest with MAM administration. (f) Micrograph depicts Ki67 labeled cells in the hippocampal DG of a control animal. *Denotes the effect of MAM; ⋄Denotes the effect of uCMS; #Denotes the antidepressants effect, by comparison of the antidepressants-treated animals with uCMS animals. *,⋄, #P<0.05, **,⋄⋄,##P<0.01, ***,⋄⋄⋄, ###P<0.001; n=5 per group. DAPI, 4',6-diamidino-2-phenylindole.
Anxiety traits frequently form part of the symptomatic profile of depressed patients and animal models of depression.8, 22, 23, 24 Ablation of hippocampal neurogenesis was here found to prevent the spontaneous reinstatement of baseline emotional states in animals previously exposed to uCMS; this was evident in two tests of anxiety-like behavior, EPM (F6–63=4.122; P=0.0015, post-hoc P<0.01, Figure 2e) and NSF (F6–63=8.932; P<0.0001, post-hoc P<0.001, Figure 2f). Both imipramine and fluoxetine normalized the anxious phenotype induced by uCMS, but whereas the therapeutic effects of fluoxetine were compromised by MAM administration (t18=1.739; P=0.0495 in the EPM, Figure 2e; and t18=1.893; P=0.0373 in the NSF, Figure 2f), those of imipramine were not (P>0.05 in both tests, Figures 2e and f).
Complaints of cognitive impairment are common among depressed subjects. Stress is known to dysregulate a number of cognitive functions that depend on the structural integrity of the hippocampus, prefrontal cortex and reciprocal connections between these two regions. Here, we show that arrest of neurogenesis prevents spontaneous improvements in spatial behavioral flexibility (F6–63=2.309, P<0.0445, post-hoc P<0.05, Figure 2g) and spatial working memory (F2–33=3.768, P=0.034, post-hoc P<0.05, Figure 2h) for up to 4 weeks after cessation of the uCMS paradigm. Impairments induced by uCMS were reversed by both imipramine and fluoxetine (post-hoc P<0.05 in both tests; Figures 2g and i), but interestingly, whereas fluoxetine failed to restore working memory when it was coadministered with MAM, the cognition-improving efficacy of imipramine did not depend on active neurogenesis (F3–31=3.081, P=0.041 post-hoc P<0.05, Figure 2j). Imipramine treatment elicited a strong pro-gliogenic effect (F6–28=6.079; P=0.0004, post-hoc P<0.01, Figure 3b), as compared with fluoxetine which was more effective at promoting differentiation of newly born cells into neurons rather than astroglia (F6–28=17.35, P<0.0001, post-hoc P<0.001). Consistently, blockade of neurogenesis with MAM did not interfere with the ability of imipramine to improve working memory (post-hoc P>0.05, Figure 2j).
uCMS induces sustained neuromorphological changes in newly born hippocampal neurons
Mood, anxiety and cognitive performance are regulated by dynamic alterations in synaptic and dentritic structure and function.8, 25, 26 The present study shows that the neuromorphological changes that we previously observed immediately after uCMS exposure8 are fully reversed after a 4-week period of recovery (Figure 4a). In fact, no differences were observed in the dendritic morphology and spine density of mature dentate gyrus neurons between controls and animals previously submitted to uCMS (Figure 4a). However, using the novel Immuno-Golgi method to distinguish between newly born and preexisting neurons,19 we observed that uCMS affects neither dendritic extension (Figures 4b and c) nor overall spine density (Figure 4d) in newborn neurons; rather, our analysis revealed that new cells generated during spontaneous and AD-induced recovery from uCMS have an unusually greater density of thin spines (F4–70=5.103, P=0.0011, post-hoc P<0.05; Figures 4e and f). As thin spines are a common feature in conditions of reduced cognitive function,27, 28 these findings indicate that uCMS leaves persistent morphological and behavioral scars. Interestingly MAM treatment mimicked sustained spines alterations in BrdU+ neurons produced by uCMS exposure, which were reversed by ADs coadministration (Supplementary Figure S7).
Exposure to unpredictable chronic mild stress (uCMS) causes long-term neuromorphological alterations in newborn granule neurons. (a) Structural changes in the dendritic arborization of preexisting granule neurons in the hippocampal dentate gyrus (DG), analyzed with the Golgi-Cox impregnation method, induced by uCMS exposure are reverted at long-term (4 weeks after uCMS exposure). (b–d) uCMS exposure does not have long-term impact on the dendritic arborization (b, c), neither in the global spine density of newborn neurons in the hippocampal DG (d), analyzed with the Immuno-Golgi method (co-labeling of BrdU and Golgi-Cox staining). (e, f) Newborn granule neurons of rats exposed to uCMS 4 weeks before, present increased percentage of thin spines; these alterations are attenuated in the animal groups treated with both antidepressants (e, f). ⋄Denotes the effect of uCMS; ⋄P<0.05; n=10–15 per group.
Discussion
Overall, the present results show that the appropriate incorporation of newly born hippocampal cells in preexisting neural networks is essential for spontaneous recovery from depression-associated emotional and cognitive impairments in rats; moreover, these processes are important for long-term maintenance of the behavioral homeostasis achieved through the use of ADs.
Indeed, hippocampal cell proliferation arrest by MAM on untreated animals caused a 70% reduction on neurogenesis and gliogenesis, similarly to the anti-proliferative effects produced by uCMS exposure, which were causally associated with long-lasting emotional and cognitive disabilities. Remarkably, MAM treatment in naive animals produced behavioral deficits resembling those manifested after chronic exposure to stress. In this regard, it is important to note that such emotional and cognitive impairments emerged only 4 weeks after cessation of MAM treatment and were not manifested shortly after neurogenesis ablation, as we have showed previously.8 Considering the late manifestation of these behavioral disabilities, it is inferable that continuous proliferation and complete circuitry integration of new neurons and glial cells, a process that takes 4–6 weeks in rodents, is necessary for the maintenance of emotional and cognitive homeostasis.
In line with the idea that continuous hippocampal cell proliferation is relevant for the spectrum of depressive symptoms, is the present observation that the ability of ADs to reverse emotional and cognitive impairments relies on their potential to restore hippocampal neuro- and glio-genesis. An interesting finding is that different classes of ADs have distinct impact on newborn cells-fate, that are reflected in different behavioral effects. As data suggests, noradrenaline reuptake inhibitors (imipramine) can ameliorate anxiety and cognitive deficits induced by stress independently of ongoing neurogenesis, whereas the anxiolytic and pro-cognitive efficacy of serotonin reuptake inhibitors (fluoxetine) require uninterrupted neurogenic processes.
These findings are consistent with earlier demonstrations of the essential role of neurogenesis in the regulation of anxiety behavior8, 9, 24, 29 and cognitive function30, 31 although the distinct contributions of serotoninergic and noradrenergic pathways to this regulation have not been previously reported. In light of recent data suggesting that astrocytic dysfunction and glial pathology have an important role in the regulation of emotional and cognitive behavior,12, 32, 33 our observation that imipramine treatment promotes the generation and differentiation of new hippocampal cells into astrocytes may explain its strong ability to counteract MAM treatment; this is consistent with the fact that, norepinephrine, in contrast to serotonin, can directly activate the resident pool of progenitor cells and stimulate neurogenic, as well as gliogenic processes.34
In addition, the present results indicate that reestablishment of synaptic connections in hippocampal networks is a likely prerequisite for the spontaneous and pharmacological restoration of normal emotional and cognitive states. Studies demonstrating the fast behavioral actions of ketamine, an NMDA receptor antagonist, support this view.35 However, we also reveal, for the first time, the occurrence of persistent alterations in spine morphology in neurons that escaped the stress- or MAM-induced hippocampal arrest that might be of relevance for future exposures to stressful conditions. Overall, our findings suggest that slower neuroplastic changes, involving neurogenesis and complex dynamic remodeling of neuro-glial networks, appear to have an important role in determining the extent of recovery from, and eventual relapse of, depressive symptoms.
In summary, this work suggests that cytogenic alterations are relevant but cannot account for the entire behavioral phenotypic recover after the remission from depressive-like behavior, as some behavioral alterations do not correlate with variations in neuro- and glio-genesis. Thus, most likely the incorporation of newly born cells into preexistent circuits impact, in combination with complementary neuroplastic and neuroendocrine alterations, on cortico-limbic circuitries involved in the remission from depressive symptomatology.
References
Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA . Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 2011; 476: 458–461.
Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C et al. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry 2011; 16: 1177–1188.
Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K . High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 2007; 317: 819–823.
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003; 301: 805–809.
Holick KA, Lee DC, Hen R, Dulawa SC . Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology 2008; 33: 406–417.
Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry 2006; 11: 514–522.
Vollmayr B, Simonis C, Weber S, Gass P, Henn F . Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry 2003; 54: 1035–1040.
Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 2009; 14: 764–773, 739.
David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 2009; 62: 479–493.
Jayatissa MN, Henningsen K, West MJ, Wiborg O . Decreased cell proliferation in the dentate gyrus does not associate with development of anhedonic-like symptoms in rats. Brain Res 2009; 1290: 133–141.
Sahay A, Hen R . Adult hippocampal neurogenesis in depression. Nat Neurosci 2007; 10: 1110–1115.
Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS . Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry 2007; 62: 496–504.
Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 2010; 15: 501–511.
Czeh B, Di Benedetto B . Antidepressants act directly on astrocytes: Evidences and functional consequences. Eur Neuropsychopharmacol 2012 doi:10.1016/j.euroneuro.2012.04.017.
Bessa JM, Mesquita AR, Oliveira M, Pêgo JM, Cerqueira JJ, Palha JA et al. A trans-dimensional approach to the behavioral aspects of depression. Front Behav. Neurosci 2009; 3: 1.
Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E . Neurogenesis in the adult is involved in the formation of trace memories. Nature 2001; 410: 372–376.
Bruel-Jungerman E, Laroche S, Rampon C . New neurons in the dentate gyrus are involved in the expression of enhanced long- term memory following environmental enrichment. Eur J Neurosci 2005; 21: 513–521.
Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N . The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci 2007; 27: 2781–2787.
Pinto L, Mateus-Pinheiro A, Morais M, Bessa JM, Sousa N . Immuno-golgi as a tool for analyzing neuronal 3D-dendritic structure in phenotypically characterized neurons. PloS one 2012; 7: e33114.
Willner P . Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 2005; 52: 90–110.
Detke MJ, Johnson J, Lucki I . Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol 1997; 5: 107–112.
Heim C, Nemeroff CB . The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 2001; 49: 1023–1039.
Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274: 1527–1531.
Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry 2009; 14: 959–967.
Guan J-S, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 2009; 459: 55–60.
Mucha M, Skrzypiec AE, Schiavon E, Attwood BK, Kucerova E, Pawlak R . Lipocalin-2 controls neuronal excitability and anxiety by regulating dendritic spine formation and maturation. Proc Natl Acad Sci USA 2011; 108: 18436–18441.
Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W et al. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci 2010; 30: 7507–7515.
Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex 2006; 16: 313–320.
Kirby ED, Friedman AR, Covarrubias D, Ying C, Sun WG, Goosens KA et al. Basolateral amygdala regulation of adult hippocampal neurogenesis and fear-related activation of newborn neurons. Mol Psychiatry 2012; 17: 527–536.
Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P et al. Spatial relational memory requires hippocampal adult neurogenesis. PloS one 2008; 3: e1959.
Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 2009; 325: 210–213.
Banasr M, Dwyer JM, Duman RS . Cell atrophy and loss in depression: reversal by antidepressant treatment. Curr Opin Cell Biol 2011; 23: 730–737.
Tanaka SC, Shishida K, Schweighofer N, Okamoto Y, Yamawaki S, Doya K . Serotonin affects association of aversive outcomes to past actions. J Neurosci 2009; 29: 15669–15674.
Jhaveri DJ, Mackay EW, Hamlin AS, Marathe SV, Nandam LS, Vaidya VA et al. Norepinephrine directly activates adult hippocampal precursors via beta3-adrenergic receptors. J Neurosci 2010; 30: 2795–2806.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–964.
Acknowledgements
We thank M Carneiro and L Martins for technical assistance. AM-P, LP, MM and SM received fellowships from the Portuguese Foundation for Science and Technology (FCT). This work was supported by FCT (PTDC/SAU-NEU/105180/2008) and the ICVS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Mateus-Pinheiro, A., Pinto, L., Bessa, J. et al. Sustained remission from depressive-like behavior depends on hippocampal neurogenesis. Transl Psychiatry 3, e210 (2013). https://doi.org/10.1038/tp.2012.141
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2012.141
Keywords
This article is cited by
-
Glial-restricted precursors stimulate endogenous cytogenesis and effectively recover emotional deficits in a model of cytogenesis ablation
Molecular Psychiatry (2024)
-
Cell type-specific NRBF2 orchestrates autophagic flux and adult hippocampal neurogenesis in chronic stress-induced depression
Cell Discovery (2023)
-
Aberrant Hippocampal Development in Early-onset Mental Disorders and Promising Interventions: Evidence from a Translational Study
Neuroscience Bulletin (2023)
-
Adult hippocampal neurogenesis shapes adaptation and improves stress response: a mechanistic and integrative perspective
Molecular Psychiatry (2022)
-
Dissecting the role of adult hippocampal neurogenesis towards resilience versus susceptibility to stress-related mood disorders
npj Science of Learning (2022)