Abstract
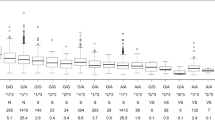
Using population pharmacokinetic analysis (PPK), we attempted to identify predictors of S-warfarin clearance (CL(S)) and to clarify population differences in S-warfarin pharmacokinetics among a cohort of 378 African American, Asian and white patients. Significant predictors of CL(S) included clinical (age, body weight and sex) and genotypic (CYP2C9*2,*3 and *8) factors, as well as African American ethnicity, the median CL(S) being 30% lower in the latter than in Asians and whites (170 versus 243 and 250 ml h−1, P<0.01). The plasma S-warfarin (Cp(S)) time courses following the genotype-based dosing algorithms simulated using the PPK estimates showed African Americans with CYP2C9*1/*1 and any of the VKORC1 genotypes would have an average Cp(S) at steady state 1.5–1.8 times higher than in Asians and whites. These results indicate warfarin dosing algorithms should be evaluated in each respective ethnic population. Further study of a large African American cohort will be necessary to confirm the present findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 2005; 352: 2285–2293.
Takahashi H, Wilkinson GR, Nutescu EA, Morita T, Ritchie MD, Scordo MG et al. Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese, Caucasians and African-Americans. Pharmacogenet Genomics 2006; 16: 101–110.
The International Warfarin Pharmacogenetics Consortium. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med 2009; 360: 753–764.
Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, Gage BF et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med 2013; 369: 2283–2293.
Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T et al. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med 2013; 369: 2294–2303.
Koller EA, Roche JC, Rollins JA . Genotype-guided dosing of vitamin K antagonists [letter]. N Engl J Med 2014; 370: 1761.
Daneshjou R, Klein TE, Altman RB . Genotype-guided dosing of vitamin K antagonists [letter]. N Engl J Med 2014; 370: 1762–1763.
Pirmohamed M, Kamali F, Daly AK, Wadelius M . Oral anticoagulation: a critique of recent advances and controversies. Trends Pharmacol Sci 2015; 36: 153–163.
Nagai R, Ohara M, Cavallari LH, Drozda K, Patel SR, Nutescu EA et al. Factors influencing pharmacokinetics of warfarin in African-Americans: implications for pharmacogenetic dosing algorithms. Pharmacogenomics 2015; 16: 217–225.
Liu Y, Jeong H, Takahashi H, Drozda K, Patel SR, Shapiro NL et al. Decreased warfarin clearance associated with the CYP2C9 R150H (*8) polymorphism. Clin Pharmacol Ther 2012; 91: 660–665.
Drozda K, Wong S, Patel SR, Bress AP, Nutescu EA, Kittles RA et al. Poor warfarin dose prediction with pharmacogenetic algorithms that exclude genotypes important for African Americans. Pharmacogenet Genomics 2015; 25: 73–81.
Chan E, McLachlan AJ, Pegg M, MacKay AD, Cole RB, Rowland M . Disposition of warfarin enantiomers and metabolites in patients during multiple dosing with rac-warfarin. Br J Clin Pharmacol 1994; 37: 563–569.
Avery PJ, Jorgensen A, Hamberg AK, Wadelius M, Pirmohamed M, Kamali F et al. A proposal for an individualized pharmacogenetics-based warfarin initiation dose regimen for patients commencing anticoagulation therapy. Clin Pharmacol Ther 2011; 90: 701–706.
Ohara M, Takahashi H, Lee MT, Wen MS, Lee TH, Chuang HP et al. Determinants of the over-anticoagulation response during warfarin initiation therapy in Asian patients based on population pharmacokinetic-pharmacodynamic analyses. PLoS One 2014; 9: e105891.
Scordo MG, Pengo V, Spina E, Dahl ML, Gusella M, Padrini R . Influence of CYP2C9 and CYP2C19 genetic polymorphisms on warfarin maintenance dose and metabolic clearance. Clin Pharmacol Ther 2002; 72: 702–710.
Takahashi H, Kashima T, Kimura S, Muramoto N, Nakahata H, Kubo S et al. Determination of unbound warfarin enantiomers in human plasma and 7-hydroxywarfarin in human urine by chiral stationary-phase liquid chromatography with ultraviolet or fluorescence and on-line circular dichroism detection. J Chromatogr B Biomed Sci Appl 1997; 701: 71–80.
Perera MA, Cavallari LH, Limdi NA, Gamazon ER, Konkashbaev A, Daneshjou R et al. Genetic variants associated with warfarin dose in African-American individuals: a genome-wide association study. Lancet 2013; 382: 790–796.
Hruska MW, Frye RF, Langaee TY . Pyrosequencing method for genotyping cytochrome P450 CYP2C8 and CYP2C9 enzymes. Clin Chem 2004; 50: 2392–2395.
Beal SL, Sheiner LB . NONMEM user’s guides. NONMEM Project Group, University of California: San Francisco, 1994.
Hamberg AK, Dahl ML, Barban M, Scordo MG, Wadelius M, Pengo V et al. A PK-PD model for predicting the impact of age, CYP2C9, and VKORC1 genotype on individualization of warfarin therapy. Clin Pharmacol Ther 2007; 81: 529–538.
Parke J, Holford NH, Charles BG . A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Programs Biomed 1999; 59: 19–29.
Takahashi H, Wilkinson GR, Caraco Y, Muszkat M, Kim RB, Kashima T et al. Population differences in S-warfarin metabolism between CYP2C9 genotype-matched Caucasian and Japanese patients. Clin Pharmacol Ther 2003; 73: 253–263.
Hernandez W, Aquino-Michaels K, Drozda K, Patel S, Jeong Y, Takahashi H et al. Novel single nucleotide polymorphism in CYP2C9 is associated with changes in warfarin clearance and CYP2C9 expression levels in African Americans. Transl Res 2015; 165: 651–657.
Daneshjou R, Gamazon ER, Burkley B, Cavallari LH, Johnson JA, Klein TE et al. Genetic variant in folate homeostasis is associated with lower warfarin dose in African Americans. Blood 2014; 124: 2298–2305.
Gong IY, Schwarz UI, Crown N, Dresser GK, Lazo-Langner A, Zou G et al. Clinical and genetic determinants of warfarin pharmacokinetics and pharmacodynamics during treatment initiation. PLoS One 2011; 6: e27808.
Limdi NA, Wadelius M, Cavallari L, Eriksson N, Crawford DC, Lee MT et al. Warfarin pharmacogenetics: a single VKORC1 polymorphism is predictive of dose across 3 racial groups. Blood 2010; 115: 3827–3834.
Limdi NA, Brown TM, Yan Q, Thigpen JL, Shendre A, Liu N et al. Race influences warfarin dose changes associated with genetic factors. Blood 2015; 126: 539–545.
Acknowledgements
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (KAKENHI C, 20590548), the Department of Health, Taiwan (DOH101-TD-PB-111-TM005), and an American Heart Association Midwest Affiliate Spring 2010 Grant-in Aid (10GRNT3750024).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kubo, K., Ohara, M., Tachikawa, M. et al. Population differences in S-warfarin pharmacokinetics among African Americans, Asians and whites: their influence on pharmacogenetic dosing algorithms. Pharmacogenomics J 17, 494–500 (2017). https://doi.org/10.1038/tpj.2016.57
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2016.57