Abstract
The treatment planning in radiation therapy (RT) can be arranged to combine benefits of computed tomography (CT) and magnetic resonance imaging (MRI) together to maintain dose calculation accuracy and improved target delineation. Our aim is study the dosimetric impact of uniform relative electron density assignment on IMRT treatment planning with additional consideration given to the effect of a 1.5 T transverse magnetic field (TMF) in MR-Linac.
A series of intensity modulated RT (IMRT) plans were generated for two representative tumor sites, pancreas and prostate, using CT and MRI datasets. Representative CT-based IMRT plans were generated to assess the impact of different electron density (ED) assignment on plan quality using CT without the presence of a 1.5 T TMF. The relative ED (rED) values used were taken from the ICRU report 46. Four types of rED assignment in the organs at risk (OARs), the planning target volumes (PTV) and in the non-specified tissue (NST) were considered. Dose was recalculated (no optimization) using a Monaco 5.09.07a research planning system employing Monte Carlo calculations with an option to include TMF. To investigate the dosimetric effect of different rED assignment, the dose-volume parameters (DVPs) obtained from these specific rED plans were compared to those obtained from the original plans based on CT.
Overall, we found that uniform rED assignment results in differences in DVPs within 3% for the PTV and 5% for OAR. The presence of 1.5 T TMF on IMRT DVPs resulted in differences that were generally within 3% of the Gold St for both the pancreas and prostate. The combination of uniform rED assignment and TMF produced differences in DVPs that were within 4–5% of the Gold St. Larger differences in DVPs were observed for OARs on T2-based plans.
The effects of using different rED assignments and the presence of 1.5 T TMF for pancreas and prostate IMRT plans are generally within 3% and 5% of PTV and OAR Gold St values. There are noticeable dosimetric differences between the CT- and MRI-based IMRT plans caused by a combination of anatomical changes between the two image acquisition times, uniform rED assignment and 1.5 T TMF.
Export citation and abstract BibTeX RIS
Introduction
A radiation treatment plan is often generated using both CT and MRI imaging modalities in order to pool the advantages of both imaging modalities together. The utility of using CT in radiation therapy (RT) includes the electron density information used in dose calculations and reference image generation for patient position verification based on in-room x-ray imaging, while the advantages of magnetic resonance imaging (MRI) lie in its high soft tissue contrast and functional/biological imaging capabilities. However, the lack of electron density (ED) information, geometric distortions (caused by magnetic inhomogeneities, nonlinear gradients, and susceptibility/chemical shifts associated with MRI) are among the limiting factors in the use of MRI as the primary imaging modality for radiation therapy (RT) planning (Paulson et al 2015). The integrated MRI with linear accelerator delivery systems (MR-Linac) or with Co60 teletherapy system (ViewRay Inc.) make the sole use of MRI in radiation oncology even more advantageous for RT planning (Lagendijk et al 2008, Fallone 2014, Keall et al 2014, Mutic and Dempsey 2014). Several groups have presented different techniques to overcome the lack of ED information necessary for MR-based RT planning of the brain (Kristensen et al 2008, Stanescu et al 2008, Jonsson et al 2010, Uh et al 2014, Andreasen et al 2015), head and neck (Jonsson et al 2010, Karotki et al 2011, Hsu et al 2013, Korsholm et al 2014), thorax (Jonsson et al 2010), vesica (Korsholm et al 2014), pelvis (Korsholm et al 2014) and prostate (Lee et al 2003, Chen et al 2004a, 2004b, Eilertsen et al 2008, Jonsson et al 2010, Lambert et al 2011, Dowling et al 2012, Kapanen et al 2013, Korhonen et al 2013, 2014, Korsholm et al 2014).
Atlas based techniques have been proposed to overcome MRI's lack of ED by creating synthetic CTs (sometimes referred to as pseudo CT) for accurate dose calculation. These techniques utilize co-registered pair of MRI and CT scans registered to the patient's MRI in order to create a synthetic CT (Dowling et al 2012, Uh et al 2014). For example, Dowling et al developed a prostate MRI and CT atlas and found dosimetric differences of their synthetic CT to be within 2% of the planning CT. Techniques like Dowling's are advantageous given their ability to automatically segment structures important for planning; however, atypical patient anatomy introduces additional registration difficulties that can prolong RT planning.
Voxel based techniques offer an alternative to atlas based methods by directly converting the MRI signal intensity (IMRI) to hounsfield unit (HU), ED or relative ED (rED) (Hsu et al 2013, Jonsson et al 2013, Kapanen and Tenhunen 2013, Rank et al 2013). These methods typically consist of a workflow utilizing a set of co-registered MRI and CT datasets, but differ in the conversion method used: probabilistic tissue classification maps (Hsu et al 2013), high dimensional discriminant analysis (Rank et al 2013), Gaussian mixture regression model (Jonsson et al 2013) and IMRI-to-HU integral mode (Kapanen and Tenhunen 2013). The resulting synthetic CT dataset allows for targets and organs at risk (OARs) to be delineated for RT planning (Hsu et al 2013, Edmund et al 2014). The dosimetric accuracy of such methods as Korhonen et al reported was less than 1% between dual mode voxel-based synthetic CTs and planning CT for prostate RT (Kapanen and Tenhunen 2013, Korhonen et al 2014). Other methods have reported dosimetric differences between synthetic and planning CTs to be no larger than 2–3%. These techniques are amenable to automatic segmentation workflows and can utilize multiple MRI images for the conversion of air, bone and different soft tissue types that are subsequently assigned a HU and/or rED value. However, variations in both image intensity between different modalities and image intensity uniformity can challenge the accuracy of intensity-based segmentation and registration approaches used in voxel-based classification methods.
Another technique to overcome the lack of ED in MRI involves assigning a bulk rED to manually contoured targets and OARs. Some authors derive their rED values from the international commission on radiation units and measurements (ICRU) Report 46 (White and Wilson 1992). For example, Jonsson et al considered ICRU Report 46 rED values for cranium, femoral bone, lung, and soft tissue. Other authors have considered other rED values such as water only or a mixture of water, air and bone. For example, Eilertsen et al and Karotiki et al consider homogenous water, air and bone rED assignments in prostate and head and neck plans, respectively. Both authors reported dosimetric differences of <2% and 4–5% for target and OAR, respectively, between uniform rED and planning CT. In general, studies using uniform rED assignment report dosimetric differences ⩽2% and ⩽5% for target and OAR, respectively (Lee et al 2003, Chen et al 2004a, 2004b, Eilertsen et al 2008, Kristensen et al 2008, Stanescu et al 2008, Jonsson et al 2010, Karotki et al 2011, Lambert et al 2011, Dowling et al 2012, Hsu et al 2013, Kapanen et al 2013, Korhonen et al 2013, 2014, Korsholm et al 2014, Uh et al 2014, Andreasen et al 2015). Despite being laborious and subjective in nature, this technique gives treatment planners a straight-forward methodology using MRI datasets they are familiar with for target and OAR delineation. However, this approach is challenged by the subjectivity of manual contouring.
The exclusive use of MRI for treatment planning in RT is advantageous for hybrid MRI-radiation delivery systems like MR-Linac and ViewRay systems but it comes with some complications. Such systems would offer improved targeting during RT for many tumor sites including prostate and pancreas, while potentially leading to improved treatment outcome. The dosimetric effects of bulk rED assignment is normally tumor-site specific and the study on the impact of rED assessment on RT plans for pancreatic cancer has not been reported, at least not to our knowledge (Rasch et al 1999, Dalah et al 2014). Nor have the combined effects of bulk rED assignment and magnetic field on pancreas and prostate IMRT plan quality been investigated. In this study, we examine the effects of bulk ED assignment on plan quality for representative pancreas and prostate cancers treated with step-and-shoot IMRT. We will examine the plan quality for treatments without and with a 1.5 T transverse magnetic field (TMF) passing through the patient, which will be useful in comparing and contrasting the plan quality of the MR-Linac to conventional treatments without a large magnetic field present. Lastly, comparisons between CT- and T2-based treatment plans with a 1.5 T TMF present will also be considered since planning on MRI images will be important for MRI-guided RT (MRIgRT) treatments.
Methods
Our study is broken up into four parts, where the first two sections investigate the effects of bulk rED assignment on a representative pancreas and prostate case without and with a TMF present. The third part investigates the inter-patient variation in CT- and MRI-based dose-volume parameters (DVPs) in five randomly selected pancreas and prostate cases. The final section investigates the differences between CT- and T2-based plans that would be used for patient treatment in the presence of a 1.5 T TMF.
Description of CT and MRI datasets
This planning study utilized both CT and MRI T2 datasets. On the CT-based plans the pancreas tumor was contoured on both images of 0%–50% phase 4D CTs and a pancreas planning target volume (PTV) generated by tracing the contours with 10 mm margins and transferred to a 3D CT for planning. On axial T2 MR-based (triggered at 50% phase) plans, the pancreas PTV was transferred from the CT to the registered T2 image dataset. For the CT- and axial T2-based prostate plans, the PTV was generated with a 5 mm margin on superior, inferior, lateral and anterior directions and a 3 mm margin on the posterior side of the prostate contour. Delineation of all pancreas and prostate OARs was performed on the MRI T2 dataset for planning.
Geometric distortion between the CT and T2 was assessed by inspecting differences in the external contour between CT and T2. We verified that each patient's MRI images did not have severe 'warping' of or large differences between the external contours primarily along the lateral and posterior portions of the patient surface. Specific information in regards to image acquisition and controls for respiratory motion, off-resonance effects, gradient nonlinearity distortions and MRI sequence parameters used at our institution have been previously described by Paulson et al (2015).
RT treatment planning
Pancreas and prostate IMRT plans were generated with the dose-volume criteria shown in table 1 using a research planning system (Monaco v5.09.07a, Elekta AB.) that utilizes a graphics processing unit (GPU) based Monte Carlo dose calculation engine and considers the effects of a TMF to the beam central axis (CAX) (Paudel et al 2015). All IMRT plans were calculated with 6 MV photons using 0.2 cm grid spacing and a 1% statistical uncertainty per plan. Information pertaining to the pancreas and prostate treatment plans (e.g. number of fractions, number of segments, total number of beams, etc) is shown in table 2. Additionally, a collimator angle of 0° was used for planning on an Elekta Infinity linear accelerator with a multi-leaf collimator (MLC) width of 0.5 cm. Plans generated based on the original planning CTs without rED assignment and the absence of a TMF were considered as the gold standard (Gold St) in this study. Dose reoptimization was performed on select T2-based plans in the fourth part of the study using the same cost function and weights as in the Gold St plans. The prescription dose on the optimized plans was rescaled to cover 95% of the PTV.
Table 1. Pancreas (left two columns) and prostate (right two columns) dose-volume criteria used in treatment planning.
| Pancreas cases | Prostate cases | ||
|---|---|---|---|
| PTVpancreas | D95 ⩾50.4 Gy | PTVprostate | D95 ⩾ 75.6 Gy |
| Duodenum | Dmax ⩽ 53 Gy | Bladder | V70 ⩽ 15% |
| Small bowel | Dmax ⩽ 53 Gy | V45 ⩽ 50% | |
| V45 ⩽ 25% | Rectum | V70 ⩽ 15% | |
| Large bowel | Dmax ⩽ 53 Gy | V45 ⩽ 50% | |
| V45 ⩽ 25% | R-fem. head | Dmax ⩽ 52.5 Gy | |
| Stomach | Dmax ⩽ 53 Gy | V50 ⩽ 2% | |
| V45 ⩽ 25% | L-fem. head | Dmax ⩽ 52.5 Gy | |
| L-kidney | V15 ⩽ 15% | V50 ⩽ 2% | |
| R-kidney | V15 ⩽ 15% | Large bowel | V60 ⩽ 2% |
| Liver | Dmean ⩽ 28 Gy | Small bowel | V50 ⩽ 2% |
| V30 ⩽ 30 Gy | Pubic bone | V60 ⩽ 30% |
Abbreviations: D95—dose receiving 95% of the target volume; V45 ⩽ 25%—volume receiving ⩾ 45 Gy; R-fem. head = right femoral head; and L-fem. head—left femoral head.
Table 2. Information pertaining to the pancreas and prostate step-and-shoot IMRT plans used in this report.
| Anon. Pt | # Fx | # Seg. | # Beams |
|---|---|---|---|
| Panc1 | 28 | 55 | 5 |
| Panc2 | 28 | 28 | 5 |
| Panc3 | 28 | 28 | 5 |
| Panc4 | 28 | 22 | 5 |
| Panc5 | 28 | 58 | 5 |
| Prost1 | 42 | 57 | 8 |
| Prost2 | 42 | 40 | 7 |
| Prost3 | 42 | 60 | 7 |
| Prost4 | 42 | 56 | 9 |
| Prost5 | 42 | 42 | 7 |
Note: The table lists the treatment plan parameters for anonymized pancreas and prostate patients (Anon. Pt) used in our study (e.g. total number of fractions (# Fx), total number of plan segments (# Seg), and total number of beams in the plan (# Beams)).
The DVPs obtained from the CT- and T2-based uniform rED plans were compared against those from the Gold St plan (table 3 and 4). The following DVPs were considered: Dose receiving 95% of the target volume (D95%) and D5% for PTV, the mean dose (Dmean), the maximum dose to 0.03 cubic centimeters (cc) (Dmax), and volume receiving ⩾15 Gy (V15—kidneys), V30 (liver), V45 (duodenum, small bowel, stomach, bladder and rectum) and V70 (bladder and rectum) for select OARs. Dosimetric comparisons between the forced rED plans and Gold St will consider the relative difference (i.e. the ratio of the difference in absolute dose between the plan and the Gold St to the absolute dose from the Gold St) except for Vx parameters (x = 15, 30 or 45), which will consider the percentage point difference. Note that a negative point or dose difference is indicative of a value that is less than the corresponding Gold St value by an amount equal to the point or dose difference. Dose-volume histograms (DVHs) will also be used to visualize the dose differences between various rED assignments.
Table 3. List of cases used to assess the dosimetric effect of various relative electron density (rED) assignments on IMRT plan quality.
| Modality | Plan type | ED forced | NST | PTV |
PTV |
B-field | Optimization |
|---|---|---|---|---|---|---|---|
| CT | Gold St | None | Not present | Yes | |||
| CT_D1_B0_O0 | Type1 | 1.022 | 0.980 | 1.031 | Not present | No | |
| CT_D2_B0_O0 | Type2 | 1.000 | 0.980 | 1.031 | Not present | No | |
| CT_D3_B0_O0 | Type3 | 1.000 | 1.000 | 1.000 | Not present | No | |
| CT_D4_B0_O0 | Type4 | 1.022 | 0.950 | 0.970 | Not present | No | |
| CT_D0_B1_O0 | None | Present | No | ||||
| CT_D1_B1_O0 | Type1 | 1.022 | 0.980 | 1.031 | Present | No | |
| T2 | T2_D1_B1_O0 | Type1 | 1.022 | 0.980 | 1.031 | Present | No |
| T2_D1_B1_O1 | Type1 | 1.022 | 0.980 | 1.031 | Present | Yes |
Note: The convention used in the Plan type column is that a CT plan with rED assignment of type1 (D1) without a magnetic field (B0) and no optimization (O0) will be labeled as CT_D1_B0_O1. A T2 plan with rED type1 with a magnetic field and with optimization would be labeled as T2_D1_B1_O1. Our assessment also considers the effects of optimization and a magnetic field passing through the patient. The a for pancreas and prostate PTVs is a reminder that the values 0.980 and 1.031 are average electron densities taken from the representative pancreas and prostate planning CT. The analysis of interpatient deviations in dose-volume parameters will take average rED values taken from each patient's planning CT. The type4 assignment will consider lower rED values by the same proportional amount as listed in the table.
Table 4. List of relative electron density for all organs at risk using pancreas and prostate IMRT treatment planning.
| Organ at risk | Relative electron density | Organ was delineated for which type of IMRT plan? |
|---|---|---|
| Duodenum | 1.024 | Pancreas |
| Small bowel | 1.024 | Pancreas and prostate |
| Large bowel | 1.024 | Pancreas and prostate |
| Stomach | 1.024 | Pancreas |
| Kidneys | 1.042 | Pancreas |
| Liver | 1.051 | Pancreas |
| Urinary bladder (filled) | 1.027 | Prostate |
| Rectum | 1.024 | Prostate |
| Pubic bone | 1.27 | Prostate |
| Penile bulb | 1.27 | Prostate |
| Femoral heads (30 year adult) | 1.27 | Prostate |
Abbreviations: IMRT—intensity modulated radiation therapy (IMRT). Note: The third column identifies whether the structure was delineated for the pancreas or prostate plan type. The spongey and cortical bone structures of the femoral head were contoured as a single entity not as separate compartments.
Representative pancreas and prostate cases
In the first part of our study, a series of step-and-shoot IMRT plans were generated on the planning CT of a representative pancreas and prostate case (hereafter referred to as panc2 and prost1, respectively), where the dose was recalculated (without optimization and with same monitor units (MUs) as the Gold St) on the CT datasets. The panc2 case was a 5 field IMRT plan with gantry angles 280, 325, 35, 80 and 160° and prost1 case was an 8 field IMRT plan with gantry angles 225, 275, 325, 0, 30, 70 110 and 150°. For those dose recalculated plans, a bulk rED assignment was made to structures delineated on the CT set. The rED values were taken from the ICRU Report 46 for the following OAR: kidneys, intestine (includes small and large bowel, rectum, and stomach), bladder (filled), liver and bone (prostate: adult, 30 year old skeleton femur and pancreas: skeleton-vertebral column whole C4). The mean rED from the panc2 and prost1 planning CT was used for the PTV structure for dose recalculations. The following four types of assignments were considered for the PTVs and non-specified tissue (NST) (i.e. in the regions excluding OARs and PTV but contained within the external patient contour):
- 1.Type1: rED = 1.022 (average of muscle, water, and fat from ICRU) for NST, 0.980 for pancreas PTV, and 1.031 for prostate PTV.
- 2.Type2: rED = 1.00 for NST while the rED for PTVs same as in 1.
- 3.Type3: rED of 1.000 for NST, PTV and all OAR structures.
- 4.Type4: rED = 1.022 for NST, and 0.950 and 0.970 for the pancreas and prostate PTV, respectively.
The Type4 rED assignment was chosen because the mean rED enclosed by the PTV contour was found to be less than one for some pancreas and prostate patients. The above four rED assignments were used to create 4 plans per panc2 and prost1 cases on the CT datasets (table 2, CT_D1_B0_O1 to CT_D4_B_O1). Our convention here is that a CT plan with rED assignment of type1 (D1) without a magnetic field (B0) and no optimization (O0) will be labeled as CT_D1_B0_O1. The specific planning parameters for each plan, such as rED type, 1.5 T magnetic field presence, and dose optimization are listed in table 3 (Note: table 3 shows a convention that will be useful in the last part of our study, which will use T2 images with rED type1 with a magnetic field and with optimization and would be labeled as T2_D1_B1_O1.).
Representative pancreas and prostate cases with 1.5 TMF present
The second part of the study investigates the effect on a 1.5 T TMF and rED assignment on pancreas and prostate IMRT plan quality. Two plans will be generated: (1) The dose will be recalculated with no rED assignment while a 1.5 T TMF is present (CT_D0_B1_O0); and (2) The dose will be recalculated with type1 rED assignment while a 1.5 T TMF is present (CT_D1_B1_O0). The planning parameters are the same as before and listed in table 3. Plan quality will be compared with respect to the original planning CT (Gold St).
Inter-patient variation in plan quality
In the third part of the study, we investigate the interpatient variation in DVPs in plans without (CT_D1_B0_O0) and with (CT_D1_B1_O0) a magnetic field present using the same parameters as in the first part. A total of five pancreas and prostate patients (panc1-panc4 and prost1-prost5, respectively) with step-and-shoot IMRT plans will be used. The rED values for each patient's PTV used the mean value enclosed by the PTV from their planning CT.
Differences between CT- and MR-based plans
Lastly, the differences between CT and MR-based plans were investigated using the representative pancreas and prostate plans (panc2 and prost1, respectively) with a 1.5 T TMF present. Another 2 plans were generated for the T2 datasets (table 3, T2_D1_B1_O0 and T2_D1_B1_O1) with rED assignment fixed to type1 on T2. Only one T2-based plan was generated with optimization (table 2, T2_D1_B1_O1). These T2-based plans (non-optimized and optimized) will be compared with the Gold St for both pancreatic and prostate representative cases. As before, treatment planning parameters are listed in table 3.
Results
Representative pancreas case without magnetic field
The effect of uniform rED assignment on the target D95% and D5% for CT- based pancreas IMRT plan quality were ⩽3% of Gold St (table 5). The variation in pancreas D95 was found to be at most 3.0% for CT-based IMRT plans. Changing the value of NST and PTV resulted in comparable variations in D95% (−3.0%, −2.5% and −2.7% for type1, type2 and type4 assignment, respectively). However, a homogeneous water rED (type3) assignment for all structures (i.e. target, NST and OAR) resulted in the smallest variation in D95% (0.2% higher than the Gold St). The shapes of DVH for the PTV are similar to one another for all types of rED assignment on CT-based plans (figure 1). The variation in D5% was found to vary in magnitude by −2.0%, −1.4%, 0.3% and −1.8% for type1, 2, 3 and 4 rED assignments).
Table 5. Deviation of dose volume parameters describing plan quality of various CT-based pancreas (anonymized patient panc2) IMRT plans from the original planning CT (gold standard).
| PTVpancreas | Duodenum | Small bowel | Stomach | L-kidney | R-kidney | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D95% | D5% | Dmax | Dmean | V45 | Dmax | Dmean | V45 | Dmax | Dmean | Dmean | V15 | Dmean | V15 | |
| CT_D1_B0_O0 | −3.0 | −2.0 | −3.7 | −3.0 | −2.1 | −2.3 | −1.5 | −0.2 | −1.3 | 0.1 | −1.9 | −0.9 | −1.9 | −0.9 |
| CT_D2_B0_O0 | −2.5 | −1.4 | −3.3 | −2.6 | −1.7 | −1.4 | −1.1 | −0.2 | −2.1 | 0.3 | −1.2 | −0.5 | −1.3 | −0.3 |
| CT_D3_B0_O0 | 0.2 | 0.3 | −0.1 | 0.1 | 0.0 | 0.1 | −0.5 | −0.1 | −0.5 | 0.1 | −1.1 | −0.6 | −1.1 | −0.6 |
| CT_D4_B0_O0 | −2.7 | −1.8 | −3.2 | −2.9 | −2.1 | −2.0 | −1.4 | −0.2 | −1.9 | 0.2 | −1.5 | −0.9 | −1.5 | −0.9 |
Abbreviations: L-kidney—left kidney; and R-kidney—right kidney. Note: The relative dose differences from the gold standard are displayed for the dose receiving 95% of the target volume (D95%), D5%, maximum dose (Dmax) and mean dose (Dmean). Percent point differences are shown for the volume receiving ⩾15 Gy (V15), and V45.
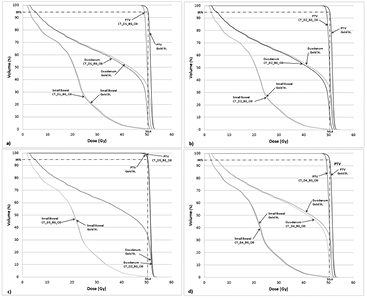
Figure 1. Pancreas IMRT dose volume histogram (DVH) for a representative case (anonymized patient panc2) for the following different relative electron density assignments: (a) CT_D1_B0_O0; (b) CT_D2_B0_O0; (c) CT_D3_B0_O0 and (d) CT_D4_B0_O0. Note each panel depicts the DVHs of the PTV, duodenum and small bowel of the given rED plan together alongside the same structures from the gold standard (i.e. planning CT).
Download figure:
Standard image High-resolution imageThe impact of uniform rED assignment on plan quality for the duodenum, small bowel, stomach and kidneys on CT-based plans was found to be <4% (table 5). The largest variation seen in pancreatic OAR was for the duodenum, where maximum variations of −3.7 and −3.0 were found for forced rED assignment type1 in Dmax and Dmean, respectively. Smaller variations were found for the homogeneous water assignment (CT_D3_B0_O0). The variations seen in the small bowel, stomach, and kidneys were found to be no larger than −2.3% compared with the Gold St.
Representative prostate case without magnetic field
A similar trend was observed for the prostate PTV, where the magnitude of variation in target IMRT plan quality from the Gold St was ⩽2% on CT-based plans (table 6). The magnitude of variation in D95% was found to be −2.0%, −1.0%, 0.7%, and −1.8% for (CT_D1_B0_O0 to CT_D4_B0_O0) rED assignments, respectively. The variation in D5% was found to vary within the same range as D95%. The smallest and largest variations in D95% were found for the homogeneous water (CT_D3_B0_O0) and type1 rED assignments (CT_D1_B0_O0), respectively. The shapes of the DVHs for the prostate PTV are similar in shape for all four rED assignments (figure 2).
Table 6. Deviation of dose volume parameters describing plan quality of various CT- and MRI-based prostate IMRT plans from the original planning CT (gold standard).
| PTVprostate | Bladder | Rectum | R-fem head | L-fem head | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D95% | D5% | V70 | V45 | Dmean | V70 | V45 | Dmean | Dmax | Dmean | Dmax | Dmean | |
| CT_D1_B0_O0 | −2.0 | −2.1 | −0.4 | −0.3 | −2.0 | −0.7 | −1.1 | −1.9 | −2.4 | −2.6 | −2.4 | −3.2 |
| CT_D2_B0_O0 | −1.0 | −1.2 | −0.3 | −0.3 | −1.6 | −0.4 | −0.8 | −1.3 | −1.0 | −2.1 | −2.1 | −2.9 |
| CT_D3_B0_O0 | 0.7 | 1.1 | 0.1 | 0.0 | 0.1 | 0.3 | 0.1 | 0.2 | 6.0 | 4.3 | 5.1 | 2.3 |
| CT_D4_B0_O0 | −1.8 | −2.0 | −0.4 | −0.3 | −2.0 | −0.6 | −1.1 | −1.8 | −4.0 | −2.8 | −2.2 | −2.9 |
Abbreviations: R-fem head—right femoral head; and L-fem head—left femoral head. Note: The relative dose differences from the gold standard are displayed for the dose receiving 95% of the target volume (D95%), D5%, maximum dose (Dmax) and mean dose (Dmean). Percent point differences are shown for the volume receiving ⩾14 Gy (V45) and V70. Dose differences values in excess of 5% have bold, italicized font.
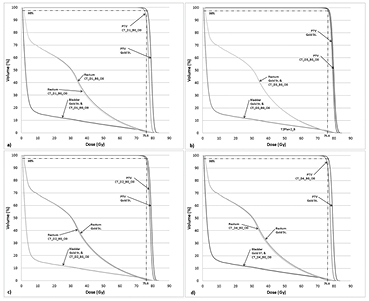
Figure 2. Prostate IMRT without 1.5 T field dose volume histogram (DVH) for a representative case (anonymized patient prost1) for the following different relative electron density assignments: (a) CT_D1_B0_O0; (b). CT_D2_B0_O0; (c) CT_D3_B0_O0 and (d) CT_D4_B0_O0. Note each panel depicts the DVHs of the PTV, rectum and bladder of the given rED plan together alongside the same structures from the gold standard (i.e. planning CT).
Download figure:
Standard image High-resolution imageThe variation in DVPs for the bladder, rectum and femoral heads was found to be ⩽6.0% for different rED CT-based plans (table 6), but the majority of differences in DVPs were <3.2%. For example, the bladder V70 and rectum Dmean was found to be no more than −0.3% and −1.9%, respectively, on CT-based plans (table 6). Both bladder and rectum DVHs are similar in shape for all CT-based plans with different rED assignments (figure 2). Homogeneous water rED assigned plans had variations larger than the Gold St, for example, the Dmax right femoral head was 6.1%. We chose to use the D1 rED assignment for the remaining parts of our analysis despite D3 yielding smaller variations in DVPs with respect to the Gold St in the pancreas case. The prostate case demonstrates the importance of assigning a reasonable value to the femoral heads in order to get boney DVP variations within 5% of the Gold St. The use of D1 assignment maintains some methodological consistency and could be important in certain situations (see discussion).
Representative pancreas and prostate case with magnetic field
When the TMF is present during dose recalculation but no re-optimization, the variation in pancreas and prostate DVPs does not exceed 3% (CT_D0_B1_O0 in tables 7(a) and (b)). This variation increases for the CT-based plans with a 1.5 T field present and type1 rED assignment (CT_D1_B1_O0) but does not exceed 3.6% for both pancreas and prostate IMRT plans. The effect of the presence of a 1.5 T TMF during dose calculation on the DVH results in curves with very similar shape (figure 3). However, a larger deviation between DVH curves is observed when forced rED assignment (type1) is used (figures 3(a)–(d) for the pancreas and prostate, respectively). The largest deviation in the prostate Dmax for the right femoral head (R-Fem Head in table 7) was 9.0% (an absolute dose difference of 24.6 Gy versus 22.3 Gy between CT_D0_B1_O0 and the Gold St, respectively).
Table 7. Deviation of dosimetric parameters describing plan quality for various CT-based pancreas (a) and prostate (b) IMRT plans with a 1.5 T field present.
| (a) | PTVpancreas | Duodenum | Small bowel | Stomach | L-kidney | R-kidney | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D95% | D5% | Dmax | Dmean | V45 | Dmax | Dmean | V45 | Dmax | Dmean | Dmean | V15 | Dmean | V15 | |
| CT_D0_B1_O0 | −0.8 | −0.7 | 0.5 | −0.8 | −0.7 | −0.1 | 0.7 | −0.2 | 1.5 | −0.7 | −2.9 | −1.1 | 1.9 | 1.6 |
| CT_D1_B1_O0 | −3.6 | −2.5 | −3.6 | −3.7 | −3.2 | −3.5 | −0.8 | −0.5 | −0.5 | −0.4 | −4.6 | −2.0 | −0.1 | 0.7 |
| (b) | PTVprostate | Bladder | Rectum | R-fem head | L-fem head | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D95% | D5% | V70 | V45 | Dmean | V70 | V45 | Dmean | Dmax | Dmean | Dmax | Dmean | |
| CT_D0_B1_O0 | −0.6 | 0.0 | −0.1 | −0.1 | −0.8 | −0.5 | 3.0 | 2.8 | 9.0 | 0.7 | 0.6 | −0.7 |
| CT_D1_B1_O0 | −2.5 | −2.1 | −0.5 | −0.4 | −2.7 | −1.2 | 1.7 | 1.0 | 3.3 | −2.4 | −2.9 | −3.3 |
Abbreviations: L-kidney—left kidney; R-kidney—right kidney; R-fem head—right femoral head; and L-fem head—left femoral head. Note: The relative dose differences from the gold standard are displayed for the dose receiving 95% of the target volume (D95%), D5%, maximum dose (Dmax) and mean dose (Dmean). Percent point differences are shown for the volume receiving ⩾15 Gy (V15), V45, and V70. Dose differences values in excess of 5% have bold, italicized font.
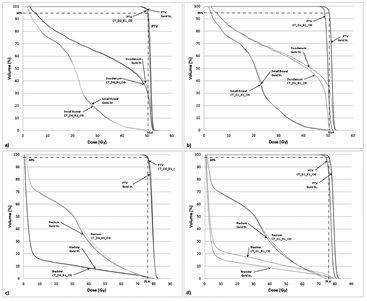
Figure 3. Pancreas (panels (a) and (b)) and prostate (panels (c) and (d)) IMRT with 1.5 T field dose volume histogram for a representative case.
Download figure:
Standard image High-resolution imageInter-patient variation in pancreas and prostate cases
A total of five pancreas and prostate plans were used to study the variation in DVPs due to uniform rED assignment and a TMF. The variation in D95% for CT-based pancreas IMRT plans is at most −3.6% (table 8(b) panc2), even for plans with a TMF present. The variation in duodenum V45 and small bowel Dmean is at most −3.1% on CT-based plans. The differences for a larger number of DVPs and more OARs show that CT-based DVPs typically vary within −2% and −5.3% of the Gold St for uniform rED plans without and with a 1.5 T TMF present, respectively.
Table 8. Variation in pancreas dosimetric parameters.
| B = 0 T | PTV | Duod | Sm bowel | Lg bowel | Stomach | L-kid | R-kid | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Panc | Plan | D95% | D5% | Dmean | V45 | Dmean | V45 | Dmean | V45 | Dmean | V45 | Dmean | V15 | Dmean | V15 |
| 1 | CT_D1_B0_O0 | −0.2 | −1.4 | −1.4 | −0.3 | −0.7 | −0.1 | −0.2 | −0.3 | 1.6 | 0.2 | −1.5 | −0.1 | −1.2 | −0.1 |
| 2 | CT_D1_B0_O0 | −3.0 | −2.0 | −3.0 | −2.1 | −1.5 | −0.2 | −2.2 | −0.2 | 0.1 | 0.0 | −1.7 | −0.8 | −1.9 | −0.9 |
| 3 | CT_D1_B0_O0 | −0.1 | −0.2 | 0.1 | 0.0 | −1.5 | −0.1 | −0.9 | −0.1 | −0.2 | 0.1 | −0.3 | 0.0 | −1.2 | −0.7 |
| 4 | CT_D1_B0_O0 | −1.1 | −1.8 | −0.4 | 0.0 | −0.6 | −0.1 | -0.4 | 0.0 | −0.8 | -0.3 | −0.7 | −0.4 | −0.6 | −0.1 |
| 5 | CT_D1_B0_O0 | −0.5 | −0.7 | −0.8 | −0.3 | −0.9 | −0.2 | −0.7 | −0.5 | −0.4 | −0.1 | −0.8 | −0.5 | −0.6 | 0.0 |
| B = 1.5 T | PTV | Duod | Sm bowel | Lg bowel | Stomach | L-kid | R-kid | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Panc | Plan | D95% | D5% | Dmean | V45 | Dmean | V45 | Dmean | V45 | Dmean | V45 | Dmean | V15 | Dmean | V15 |
| 1 | CT_D1_B1_O0 | −0.2 | 1.4 | −3.8 | −1.1 | −0.3 | 0.2 | 1.0 | −0.3 | 0.9 | 0.2 | −2.3 | 0.1 | 4.9 | 2.1 |
| 2 | CT_D1_B1_O0 | −3.6 | −2.5 | −3.7 | −3.2 | −0.8 | −0.5 | −0.3 | −0.3 | −0.4 | 0.0 | −4.6 | −2.0 | −0.1 | 0.7 |
| 3 | CT_D1_B1_O0 | −1.2 | −0.8 | −1.3 | −2.3 | 1.1 | 0.0 | −2.1 | −0.6 | 0.7 | −0.1 | −4.6 | −2.4 | −1.1 | 1.6 |
| 4 | CT_D1_B1_O0 | −1.8 | −2.7 | −5.3 | −0.4 | 3.0 | 0.3 | 3.1 | 0.0 | 0.6 | −0.1 | −2.5 | −2.5 | −1.3 | 1.7 |
| 5 | CT_D1_B1_O0 | −0.8 | −0.6 | −0.9 | −0.6 | 1.3 | −0.2 | −0.8 | −1.1 | 0.4 | −0.2 | −3.0 | −1.7 | −2.2 | −0.2 |
Abbreviations: Duod—duodenum; Sm bowel—small bowel; Lg bowel—large bowel; L-kid—left kidney; R-kid—right kidney. Note: The deviations in the parameter values for 5 pancreas patients are depicted without (top) and with (bottom) a 1.5 T magnetic field present during dose calculation. No optimization was performed for the calculation. The relative dose differences from the gold standard are displayed for the dose receiving 95% of the target volume (D95), D5, maximum dose (Dmax) and mean dose (Dmean). Percent point differences are shown for the volume receiving ⩾15 Gy (V15), V45, and V70. Dose differences values in excess of 5% have bold, italicized font.
The interpatient variation in prostate D95% and D5% was within −3.8% of the Gold St for CT-based plans (table 9). The variation was found to be no more than −3.8% and −3.5% for D95% and D5%, respectively, on cases where dose recalculation was performed without a TMF present. When a 1.5 T TMF is present, the variations tend to be slightly larger and of magnitude −5.1% and −4.5% for D95% and D5%, respectively. Similar variations were observed for the bladder, rectum and femoral heads. When a TMF is not present during dose recalculation, the variations tend to be within 6% of the Gold St. However, the magnitude of variation was within 7% of the Gold St when a TMF is present during dose recalculation. The largest difference between rED plan and the Gold St was observed in the femoral head Dmax value in both situations.
Table 9. Variation in prostate dosimetric parameters with different electron density assignment methods.
| B = 0 T | PTV | Bladder | Rectum | R-fem head | L-fem head | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prost | Plan | D95% | D5% | Dmean | V70 | V45 | Dmean | V70 | V45 | Dmean | Dmax | Dmean | Dmax |
| 1 | CT_D1_B0_O0 | −2.0 | −2.1 | −2.0 | −0.4 | −0.3 | −1.9 | −0.7 | −1.1 | −2.6 | −2.4 | −3.1 | −2.4 |
| 2 | CT_D1_B0_O0 | −3.8 | −3.5 | −3.2 | −0.5 | −0.3 | −4.0 | −2.0 | −1.7 | −3.4 | −2.2 | −3.4 | −5.3 |
| 3 | CT_D1_B0_O0 | −2.2 | −2.1 | −2.1 | −0.3 | −0.2 | −2.1 | −1.0 | −1.3 | −2.0 | −3.5 | −1.5 | −0.6 |
| 4 | CT_D1_B0_O0 | −1.8 | −1.6 | −1.7 | −0.5 | −0.5 | 2.9 | −0.3 | −0.8 | −1.9 | −2.0 | −2.0 | −5.9 |
| 5 | CT_D1_B0_O0 | −2.3 | −1.8 | −1.8 | −0.8 | −0.6 | −1.9 | −1.0 | −1.1 | −3.5 | −2.4 | −2.4 | −3.0 |
| B = 1.5 T | PTV | Bladder | Rectum | R-fem head | L-fem head | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prost | Plan | D95% | D5% | Dmean | V70 | V45 | Dmean | V70 | V45 | Dmean | Dmax | Dmean | Dmax |
| 1 | CT_D1_B1_O0 | −2.5 | −2.1 | −2.7 | −0.5 | −0.4 | 1.0 | −1.2 | 1.7 | −2.4 | 3.3 | −3.4 | −2.9 |
| 2 | CT_D1_B1_O0 | −5.1 | −4.5 | −4.7 | −0.9 | −0.5 | −2.1 | −3.0 | −0.1 | −3.7 | −2.2 | −3.2 | −7.0 |
| 3 | CT_D1_B1_O0 | −3.6 | −2.7 | −3.5 | −0.5 | −0.4 | 0.3 | −0.6 | 0.4 | 0.1 | −3.2 | −1.3 | −1.0 |
| 4 | CT_D1_B1_O0 | −3.0 | −2.3 | −2.0 | −0.8 | −0.6 | −1.4 | −1.1 | 0.0 | −2.6 | −2.0 | −2.0 | −5.3 |
| 5 | CT_D1_B1_O0 | −3.1 | −2.5 | −3.2 | −1.8 | −1.2 | 0.4 | −0.4 | 0.5 | −4.6 | −2.9 | −3.0 | −3.3 |
Abbreviations: R-fem head—right femoral head; L-fem head—left femoral head; LB—large bowel; and SB—small bowel. Note: The deviations in the parameter values for 5 prostate patients are depicted without (top) and with (bottom) a magnetic field present during dose calculation without optimization The relative dose differences from the gold standard are displayed for the dose receiving 95% of the target volume (D95), D5, maximum dose (Dmax) and mean dose (Dmean). Percent point differences are shown for the volume receiving ⩾15 Gy (V15), V45, and V70. Dose differences values in excess of 5% have bold, italicized font.
Differences between CT- and MR-based plans
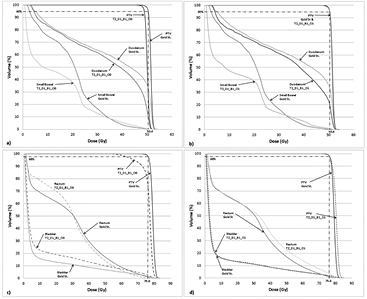
The effects on uniform rED assignment on T2-based plans (T2_D1_B1_O0 and T2_D1_B1_O1) were found to be larger (figures 4(a) and (b)) than the Gold St. When optimization of the pancreas and prostate plans was performed, target coverage was achieved without violating OAR planning constraints; however this does result in larger/smaller difference in OAR doses compared with the Gold St (figures 4(c) and (d)). These large differences in IMRT plan quality metrics was partly due to changes in anatomical shape, volume and position of the organs/tissues in between image acquisitions (figures 5 and 6). However, the DVPs for the OAR still meet the planning criteria in table 1 for both pancreas and prostate IMRT plans (i.e. T2_D1_B1_O1 in tables 10(a) and (b)). The central axis (CAX) of the beams on the T2 images pass through different tissues (i.e. beams3 and 4 in figure 5 pass through different amounts of small bowel, large bowel and NST) with slightly different rED, shape and volume that lead to small changes in absolute dose, which yield very large percent changes compared to the Gold St. For example, panc2's stomach Dmax on the Gold St was 17 Gy and 52.2 Gy on T2_D1_B1_O0 (a +183.4% difference but still clinically acceptable). Optimization of the panc2 plan reduced the stomach Dmax to 51.9 Gy, which is within clinically acceptable limits but still a +178.8% difference. A similar observation was made on prost1, where the bladder Dmean on the Gold ST were 9.2 Gy but increased to 12.7 Gy (+37.7% difference) on T2_D1_B1_O1. Re-optimization on the T2 reduced the bladder mean dose to 9.62 Gy, which is clinically acceptable but still 4.1% higher than the Gold St. Additional examples of large increases in DVP that remain within clinical acceptable range in table 1 include the 6% increase in rectum V45, which is a difference of 22.5% and 28.6% between Gold St and T2_D1_B1_O1, respectively.
Table 10. Deviation of dosimetric parameters describing plan quality for various T2-based pancreas (a) and prostate (b) IMRT plans with a 1.5 T field present.
| (a) | PTVpancreas | Duodenum | Small bowel | Stomach | L-kidney | R-kidney | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D95% | D5% | Dmax | Dmean | V45 | Dmax | Dmean | V45 | Dmax | Dmean | Dmean | V15 | Dmean | V15 | |
| T2_D1_B1_O0 | −3.1 | −2.5 | −0.1 | −6.7 | −10.0 | 1.2 | −28.0 | 1.2 | 183.4 | 227.0 | −9.3 | −4.5 | −9.1 | −0.3 |
| T2_D1_B1_O1 | 0.0 | −0.4 | −1.4 | −9.2 | −10.1 | 1.1 | −26.0 | 1.3 | 178.8 | 138.7 | −8.2 | −1.8 | −12.1 | −3.8 |
| (b) | PTVprostate | Bladder | Rectum | R-fem head | L-fem head | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D95% | D5% | V70 | V45 | Dmean | V70 | V45 | Dmean | Dmean | Dmax | Dmean | Dmax | |
| T2_D1_B1_O0 | −12.0 | −1.7 | 1.8 | 3.8 | 37.7 | −1.9 | −2.8 | 3.4 | 27.1 | 33.0 | 59.7 | 8.8 |
| T2_D1_B1_O1 | 0.3 | 1.9 | 0.0 | −0.1 | 4.1 | 3.7 | 6.1 | 8.8 | 0.5 | 24.5 | 32.6 | 14.62 |
Abbreviations: L-kidney—left kidney; R-kidney—right kidney; R-fem head—right femoral head; and L-fem head—left femoral head. Note: The relative dose differences from the gold standard are displayed for the dose receiving 95% of the target volume (D95%), D5%, maximum dose to 0.03 cubic centimeters (Dmax) and mean dose (Dmean). Percent point differences are shown for the volume receiving ⩾15 Gy (V15), V45, and V70. Dose differences values in excess of 5% have bold, italicized font.
Figure 4. Pancreas (panels (a) and (b)) and prostate (panels (c) and (d)) IMRT with 1.5 T field dose volume histogram for a representative case planned on MRI T2. Note: figures 4(b) and (d) contain DVH curves for the re-optimized plan on the T2 dataset.
Download figure:
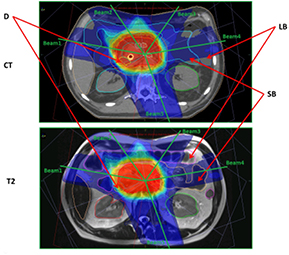
Standard image High-resolution imageFigure 5. Pancreas CT and T2 images. Abbreviations: CT—axial slice through isocenter on pancreas IMRT CT data set; T2—axial view through isocenter on prostate IMRT T2 data set; D—duodenum; LB—large bowel; SB—small bowel; Beam1—beam with central axis at a gantry angle of 280°; Beam2—beam with central axis at a gantry angle of 325°; Beam3—beam with central axis at a gantry angle of 35°; Beam4—beam with central axis at a gantry angle of 80°; and Beam5—beam with central axis at a gantry angle of 160°.
Download figure:
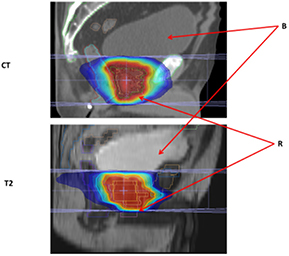
Standard image High-resolution imageFigure 6. Prostate CT and T2 images. Abbreviations: CT—sagittal view through isocenter on prostate IMRT CT data set; T2—sagittal view through isocenter on prostate IMRT T2 data set; B—bladder and R—rectum.
Download figure:
Standard image High-resolution imageDiscussion
Our study is the first to investigate the effects of uniform electron density assignment in the pancreas and the combined effects of TMF and uniform rED assignment on pancreas and prostate plan quality. Our results show using rED derived from ICRU Report 46 resulted in differences in DVPs for CT plans without a TMF that were within 5% of the Gold St (i.e. CT_D1_B0_O0 to CT_D4_B0_O0). When only a 1.5 T field is present, these differences remain largely within 5%; however some DVPs of OARs in low dose regions, where small absolute dose changes results in large percent differences, exhibit variations from the Gold St on the order of 5–9%. In general, the combined effects of uniform rED assignment and the 1.5 T TMF on plan quality results in differences in DVPs that were within 3% and 5% of the Gold St for the PTV and OARs, which agrees with the results from similar studies in the literature (Lee et al 2003, Chen et al 2004a, 2004b, Eilertsen et al 2008, Kristensen et al 2008, Stanescu et al 2008, Jonsson et al 2010, Karotki et al 2011, Lambert et al 2011, Dowling et al 2012, Hsu et al 2013, Kapanen et al 2013, Korhonen et al 2013, 2014, Korsholm et al 2014, Uh et al 2014, Andreasen et al 2015).
Even though it is difficult to isolate the effects of anatomical changes to assess its impact on plan quality; however, it is useful to investigate the combined impact of uniform rED and magnetic field on T2-based plans since the use of MRI in treatment planning with combined MRI and radiation delivery systems. Larger dose differences were found on the unoptimized T2-based plan (table 10 and figures 4(a) and (c)), where variations in PTV coverage were within 5% of the Gold St and DVP differences for OAR could be larger than 5%. These larger dose differences in OAR were partly due to anatomical changes occurring during the time between the CT and T2 image acquisition. Acceptable plans meeting dose-volume criteria (table 1) could be obtained through plan re-optimization based on T2 images (figures 4(b) and (d)), which used the same IMRT constraints from the Gold St without changing the beam orientation. The PTV was subsequently rescaled so that 95% of the PTV volume received the prescription dose, which reduced the variation in PTV plan quality DVPs to <2.0%. The use of another cost function could create a 3D dose distribution that was comparable to, if not better, than the Gold St. Performing the optimization without any changes to the plan demonstrates that the planning system could come up with an acceptable dose distribution meeting the criteria in table 1 despite that fact the OAR had shifted in position and changed in volume/size between the time of CT and MRI acquisition These differences in anatomical shape, volume and position between image acquisition times are necessary to consider in RT planning and similar establishment control methods (similar to our institutional) to reduce these differences between the two image sets is important (Paulson et al 2015).
It is well known that the presence of a magnetic field leads to an induced asymmetric point spread kernel, which causes a reduction in the build-up region and shifted/asymmetric beam penumbra (Raaymakers et al 2004). Additionally, the electron return effect (ERE) is caused by the Lorentz force sending secondary electrons at the tissue-air/lung-tissue interface back into the tissue, which leads to a buildup of dose in the skin/lung/air-cavity region (Raaijmakers et al 2005). Many of the published reports investigating the skin dose enhancements due to the magnetic field have reported substantial increases for phantom geometries (Oborn et al 2009, 2010, 2012, Keyvanloo et al 2012). The magnetic induced effects on plan quality and skin doses for IMRT/VMAT (i.e. VMAT-Volumetric-Modulated-Arc-Therapy) techniques have also been investigated for the following tumor sites: kidney, rectum, breast, lung, head and neck, and prostate (Raaijmakers et al 2007, Stam et al 2013, Tristan et al 2013, Uilkema et al 2015, Yang et al 2015, Oborn et al 2016). For example, van Heijst et al have reported that these increased skin doses are likely prohibitive for whole breast irradiation. They also report a negligible increase in skin dose for accelerated partial breast irradiation and therefore the skin dose in not clinically prohibitive for this breast treatment technique (Tristan et al 2013). Although the skin dose can be rather high in certain situations, Raaijmakers et al has demonstrated that the skin/cavity dose can be reduced by increasing the total number of beam used for plan generation (Raaijmakers et al 2007). While our analysis didn't consider the effects of ERE on the skin, previously published results from our group indicate that the presence of the 1.5 T TMF leads to a 17.0 and 20% increase in the dose to 1 cubic centimeter (D1cc) for step-and-shoot IMRT and VMAT respectively (Chen et al 2015). Although these are somewhat large percentages, the D1cc values were 22.2 and 17.2 Gy for step-and-shoot IMRT and VMAT, respectively. Therefore the increased skin doses are not likely to be clinically prohibitive for the pancreas. The results for the prostate were similar to the pancreas and therefore the skin dose increases are not likely to be clinically prohibitive.
Monte Carlo dose calculations were used in our study. This is in contrast with other investigators, the majority of whom used analytical methods of dose calculation including pencil beam algorithm (Lee et al 2003, Kristensen et al 2008, Jonsson et al 2010, 2013, Rank et al 2013); convolution/superposition (Chen et al 2004a, 2004b, Korhonen et al 2013); collapsed cone (Eilertsen et al 2008, Jonsson et al 2010, Karotki et al 2011); analytical anisotropic algorithm (Stanescu et al 2008, Lambert et al 2011, Dowling et al 2012, Hsu et al 2013, Kapanen and Tenhunen 2013, Edmund et al 2014) and Monte Carlo (Korhonen et al 2013, 2014). The consensus of all reports indicate that bulk rED assignment results in dose differences within 2% for the PTV and 5% for OARs compared to the planning CT. Our findings agree with the literature and were achieved using Monte Carlo with clinically realistic parameters (i.e. grid size and calculation uncertainty) with calculation times of less than one hour.
The values used in our analysis were derived from the ICRU Report 46 and mean rED values for the PTV for both the pancreas and prostate. We chose to use ICRU values for normal structures since those values are widely available and generally accepted. However, we acknowledge that they are population averaged values for healthy tissues unless specifically stated within the ICRU report and should be used cautiously. Workflows that utilize MRI only would find our method problematic and may need to use a volume weighted average of the uniform rED structures bounded by the PTV. However, the literatures reports studying uniform rED assignment on RT plan quality clearly suggest that acceptable plan quality can be achieved using the uniform water density assignment provided that additional structures with significantly different electron densities compared to soft tissue, like bone and air, are contoured and forced to a reasonable value (Lee et al 2003, Chen et al 2004a, 2004b, Eilertsen et al 2008, Kristensen et al 2008, Stanescu et al 2008, Jonsson et al 2010, Karotki et al 2011, Lambert et al 2011, Dowling et al 2012, Hsu et al 2013, Kapanen et al 2013, Korhonen et al 2013, 2014, Korsholm et al 2014, Uh et al 2014, Andreasen et al 2015). For example, Rank et al and Karotki et al both demonstrated improved dosimetric accuracy for uniform rED assignment for air, bone, and water compared with uniform water assignment for intercranial and head and neck cases. In cases of MRI only workflow, water, bone and air are necessary densities to force in order to achieve accurate dosimetry. Authors not comfortable with ICRU report 46 derived values should at least use, at a minimum for prostate RT, a rED assignment of water for soft tissues, spongey bone, and cortical bone (the importance of spongey and cortical bone assignment discussion follows this paragraph). While bulk rED assignment of water for soft tissues and bone for the vertebral column would be necessary for pancreas.
Some caution should be used in applying uniform rED assignment to all cases and realistic values are important for accurate dosimetry. Recently, Hoogcarspel et al demonstrated this in the inability of uniform rED assignment to generate a clinically acceptable plan for spinal bone metastases (Hoogcarspel et al 2014). However, those authors were able to generate acceptable plans by incorporating the electron density heterogeneity within the affected vertebral body into the plan. Similar findings have been reported by Korhonen et al who demonstrated the importance of assigning different rED for the cortical and spongey parts of the femoral heads in order to more accurately model the attenuation through the bone (i.e. cortical bone 1.6–2.1 rED to water and spongey bone 1.1–1.4 rED to water (Korhonen et al 2013)). The assignment of these structures was found to improve the accuracy of the dose profile around the vicinity of the prostate PTV near the femoral heads. This is why we observed higher dose differences for prostate D95 compared with the pancreas (compare tables 7 and 8).
The studies by Rank et al, Karotki et al and Korhonen et al demonstrate the importance of assigning a realistic value for bone in order to achieve more accurate dose distributions. The reason we investigated D1 assignment instead of D3 was to present a methodological consistent method of uniform rED assignment to cover a range of possible situations and that could be applicable to any tumor site. For instance, the use of D3 assignment rather than D1 will result in an inaccurate dose profile in around the pancreas PTV in situations where the target is in close proximity to the vertebral body. Additionally, the use of D3 did result in dose distributions that were higher than the Gold St in the vertebral body; however, the chosen DVPs and the DVHs displayed in our analysis aren't good metrics for showing this variation. Nonetheless, ICRU values for bone (used in D1 assignment) will result in more accurate dose distribution inside and surrounding bone compared with water (used in D3 assignment).
Conclusion
The effect of uniform electron density assignment on pancreas and prostate IMRT plan quality is well within 5% of the CT-based plan, indicating the MRI-only based IMRT planning is feasible for both pancreatic and prostate cancer. The differences observed between the CT plans with or without the magnetic field were <5%. Notable differences were found between CT- and MR-based plans due to a combination of anatomical changes, uniform rED assignment and a 1.5 T TMF, which should be considered in MR only treatment planning workflows. We recommend using ICRU report 46 for deriving appropriate rED values for planning for the pancreas and prostate since the variations in target and OAR DVPs are within values reported in similar studies found in the literature. For MR-only workflows, the rED for the PTV could be generated by taking the volume weighted average of the uniform rED structures bounded by the PTV contour.
Acknowledgments
This work is supported partially by MCW Cancer Center Fotsch Foundation and Elekta AB (Stockholm, Sweden).
Footnotes
- *
This work was present in part at the 2014 ASTRO annual meeting.