Abstract
Contrast-enhanced (CE) dual-energy (DE) x-ray breast imaging uses a low- and high-energy x-ray spectral pair to eliminate soft-tissue signal variation and thereby increase the detectability of exogenous imaging agents. Currently, CEDE breast imaging is performed with iodinated contrast agents. These compounds are limited by several deficiencies, including rapid clearance and poor tumor targeting ability. The purpose of this work is to identify novel contrast materials whose contrast-to-noise ratio (CNR) is comparable or superior to that of iodine in the mammographic energy range. A monoenergetic DE subtraction framework was developed to calculate the DE signal intensity resulting from the logarithmic subtraction of the low- and high-energy signal intensities. A weighting factor is calculated to remove the dependence of the DE signal on the glandularity of the breast tissue. Using the DE signal intensity and weighting factor, the CNR for materials with atomic numbers (Z) ranging from 1 to 79 are computed for energy pairs between 10 and 50 keV.
A group of materials with atomic numbers ranging from 42 to 63 were identified to exhibit the highest levels of CNR in the mammographic energy range. Several of these materials have been formulated as nanoparticles for various applications but none, apart from iodine, have been investigated as CEDE breast imaging agents. Within this group of materials, the necessary dose fraction to the LE image decreases as the atomic number increases. By reducing the dose to the LE image, the DE subtraction technique will not provide an anatomical image of sufficient quality to accompany the contrast information. Therefore, materials with Z from 42 to 52 provide nearly optimal values of CNR with energy pairs and dose fractions that provide good anatomical images. This work is intended to inspire further research into new materials for optimized CEDE breast functional imaging.
Export citation and abstract BibTeX RIS
For more information on this article, see medicalphysicsweb.org
1. Introduction
Contrast-enhanced (CE) dual-energy (DE) x-ray breast imaging encompasses an emerging group of modalities designed to provide quantitative functional information together with high-resolution anatomic images. This unique combination of information in a single imaging procedure represents a powerful breast imaging tool for morphological and vascular characterization of breast lesions (Chen et al 2008, Carton et al 2010a, 2010b, 2010c, Dromain et al 2012, Froeling et al 2013, Jochelson et al 2013). DE imaging is used to increase the conspicuity of exogenous radiographic imaging agents by suppressing the anatomical signal variation in the body. In the breast, this involves the suppression of the signal variation that arises from differences in soft tissue (adipose and glandular) composition across the breast. By reducing the effect of this soft tissue 'anatomical noise', it is possible to segment and quantify the signal from an exogenous imaging agent. This is performed by using two distinct energy windows (low and high) to emphasize the variation in attenuation of the various materials with energy. By employing an imaging agent whose linear attenuation k-edge lies within the energy range used, it is possible to separate the contrast material from the surrounding tissue.
K-edge imaging has been extensively applied in DE radiography to separate high atomic number (Z) contrast materials from surrounding tissue (Riederer and Mistretta 1977, Roessl and Proksa 2007, Abudurexiti et al 2010). The concepts of k-edge imaging can be visualized using two-dimensional attenuation coefficient (2DAC) maps, as shown in figure 1. The 2DAC maps plot pairs of attenuation coefficients for various materials at a particular combination of energies. The blue solid line is the linear attenuation coefficient of admixtures of adipose and glandular breast tissue ranging from 100% adipose (A) to 100% glandular (G). The red squares represent potential contrast materials with atomic numbers ranging from 1 to 80, with certain materials of interest (molybdenum (Mo), silver (Ag), iodine (I), gold (Au)) explicitly shown. Atomic number multiples of 10 are shown to serve as a guide. In general, as the atomic number increases, the attenuation coefficients at both energies increase. The discontinuities in the graph, which are drawn as dashed lines, arise from the fact that the k-edge of the indicated materials fall between the two energies. The ability to accurately separate materials at a particular combination of energies can be directly correlated to their distance from the blue A–G line on the 2DAC map. Thus, as the distance between the potential contrast material and the breast tissue values increases, the ability to separate the contrast agent from the anatomical tissue improves. As an example, figure 1 shows a 2DAC map for an energy pair of (20, 25) keV. All four materials of interest are positioned close to the breast tissue values. However, materials with Z between 43 and 46 are separated from the breast tissue values. When the energy pair is changed to (30, 35) keV as shown in figure 2, materials between 51 and 54, including iodine, are distinct from the breast tissue values. Thus, the latter energy pair is better suited for an iodinated contrast agent than the former, as expected.
Figure 1. 2DAC maps at (20, 25) keV. The blue line represents the breast glandularity values (from 0% glandular (G) to 100% glandular), while the red squares correspond to materials with atomic numbers from 1 to 80. K-edge imaging positions two spectra so that the contrast material is removed from the breast glandularity values in the 2DAC map. In this example, iodine falls very close to the breast glandularity values and therefore this energy pair would not be used for iodine imaging.
Download figure:
Standard image High-resolution imageFigure 2. 2DAC maps at (30, 35) keV. The blue line represents the breast glandularity values (from 0% glandular (G) to 100% glandular), while the red squares correspond to materials with atomic numbers from 1 to 80. K-edge imaging positions two spectra so that the contrast material is removed from the breast glandularity values in the 2DAC map. Unlike the previous example, iodine is now removed from the breast glandularity values. This energy pair could therefore be used for CEDE imaging with iodine.
Download figure:
Standard image High-resolution imageCurrently, CEDE breast imaging is performed with an iodinated contrast agent. These agents are typically small tri-iodobenzene compounds with substitutions for improved water solubility (Rode and Müller 1998). They are extremely stable and chemically inert, resisting biodegradation in vivo over long periods of time. These agents are administered intravenously to identify tumorigenic neovasculature. They are, however, plagued by several deficiencies. The non-specific nature of the contrast agent results in random vascular permeation and their relatively low molecular weight facilitates rapid renal clearance. Therefore, the percentage of the injected dose that reaches the tumor site is low because these agents are rapidly filtered by the kidneys and removed from the blood circulation. Additionally, iodinated agents have been linked to contrast media nephropathy in patients with preexisting renal insufficiency, such as diabetes mellitus (Cigarroa et al 1989, Weisberg et al 1994).
As a result, there has been much effort to develop improved imaging agents for DE radiography (Hainfeld et al 2006, Chien et al 2012, Lee et al 2012, Liu et al 2012a, 2012b, Peng et al 2012). Several materials (gold, ytterbium and tantalum) have been identified as potential alternatives. However, previous analysis and experimentation has been directed at x-ray energies (80–140 kVp) for applications other than breast radiography. Thus, there exists a need to identify materials that are specifically optimized for CEDE x-ray breast imaging. To this end, we have developed an analytical framework to compare various contrast materials quantitatively for application in CEDE breast radiography. This framework is based on the logarithmic subtraction of low-energy (LE) and high-energy (HE) images to create a DE image. Metrics are proposed to quantify the contrast and contrast-to-noise ratio (CNR) for potential imaging agents and to identify those agents that would perform better than iodine. This work is intended to inspire further research in novel contrast materials in the hope that an agent optimized for DE breast imaging may be discovered.
2. Methods
2.1. Formulation of DE signal intensity
The number of x-ray photons passing through a given material can be expressed in terms of the linear attenuation coefficient (μ) and thickness (t). In the simplistic case of a monoenergetic beam of x-rays, the photon fluence (N) after passing through a material can be written using the Beer–Lambert law as

where N0 is the initial photon fluence. The product of the linear attenuation coefficient and thickness is summed over all the materials present in the beam path. The signal intensity resulting from the photons can be expressed in terms of the logarithm of N:

In the case of CEDE x-ray breast imaging, the principal materials that contribute to the attenuation of the photons are (a)dipose, (g)landular and (c)ontrast agent. Thus, the signal intensities for both LE and HE energy photons can be written as
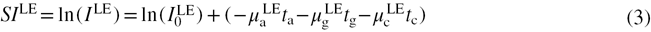
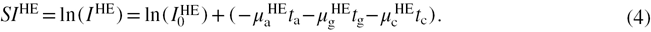
If the total thickness of tissue ( ) is assumed to consist of solely adipose and glandular components, it can be expressed as
) is assumed to consist of solely adipose and glandular components, it can be expressed as

By replacing  in equations (3) and (4), the signal intensities at each energy can be described in terms of
in equations (3) and (4), the signal intensities at each energy can be described in terms of  ,
,  and
and  .
.
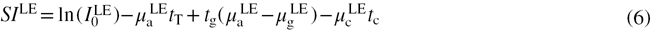
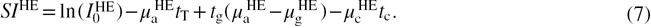
The DE signal intensity ( ) can be expressed as the weighted subtraction between the LE and HE signal intensities, using a weighting factor, W:
) can be expressed as the weighted subtraction between the LE and HE signal intensities, using a weighting factor, W:

Consequently,  can be separated into a linear combination of
can be separated into a linear combination of  ,
,  and
and  .
.
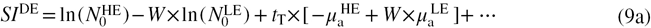
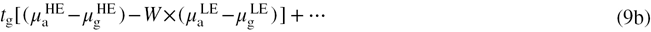

2.2. Calculation of weighting factor, contrast and CNR
The first component of SIDE (9a) is a function of the initial photon fluence and the total thickness of tissue. The initial photon fluence can be assumed to be constant across the image, given that the variation in the distance from the x-ray source to individual detector elements is small. Under mammographic compression, the total thickness of tissue varies by roughly 5% across the image of the breast, and therefore is ignored in this study (Richard et al 2006). Thus, (9a) can be assumed to be invariant across the area of the image and simply provides a constant offset to  .
.
The second component (9b) describes the relationship between  and the thickness of glandular tissue in the beam. By choosing W as
and the thickness of glandular tissue in the beam. By choosing W as

we can eliminate this dependence. The only remaining term that varies across the image is the third component (9c). This component quantifies the linear relationship between  and
and  . The DE contrast, SC, can be defined as the change in
. The DE contrast, SC, can be defined as the change in  with respect to
with respect to  :
:
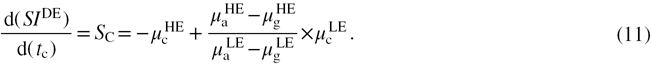
The noise (σbkg) in the background signal can be obtained from (9a), and formulated as
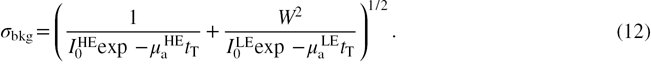
A total dose of 1.0 mGy was assumed for the LE and HE images, at a breast thickness of 5 cm. The dose distribution between the LE and HE images was allowed to vary. From (11) and (12), the CNR is calculated as

3. Results
3.1. Energy dependence of W, SC and CNR
W is calculated for LE and HE pairs between 10 and 50 keV, and plotted in figure 3. W is independent of the contrast material chosen and solely depends on the linear attenuation coefficients of adipose and glandular tissue. W assumes values between 0 and 1, and increases as the separation between the LE and HE decreases. This can be easily explained by studying the variation of the linear attenuation coefficients of adipose and glandular tissue with energy (see figure 4). As the energy increases, the difference in attenuation coefficient between the two materials decreases. Thus, W, the ratio between the differences, is least when the LE and HE are furthest apart and 1 for the trivial case when the LE is equal to the HE.
Figure 3. W calculated for energy combinations ranging from 15 to 50 keV. W ranged from 0 to 1, while steadily increasing towards the diagonal of the plot. W increases as the LE and HE are closer together.
Download figure:
Standard image High-resolution imageFigure 4. Energy dependence of the linear attenuation coefficients of glandular, adipose tissue, as well as the difference between these two materials (glandular–adipose). The difference decreases monotonically as the energy increases which lends W its defining properties (increasing monotonically, between 0 and 1).
Download figure:
Standard image High-resolution imageFigure 5 shows the energy dependence of SC for Mo (Z = 42), Ag (Z = 47), I (Z = 53) and Au (Z = 79). The graphs show that SC is only large when the LE and HE are positioned on opposite sides of the k-edge of the material. As expected, Au shows no contrast for any combination of LE and HE studied because the k-edge of gold (80 keV) lies outside of the energy range. These results demonstrate that while materials, such as gold, may have application in higher energy and temporal-subtraction imaging, their application in DE breast imaging is limited. In addition, SC shows a greater dependence on the HE value than the LE value. This can be observed in figure 6 where a contour map of SC of iodine is overlaid with arrows indicating the gradient vectors of SC. The direction of the arrows indicates the direction of the gradient vector, while the length indicates the magnitude. As the arrows have no substantial component in the LE direction, it can be deduced that the location of the HE has a greater influence on SC than the location of the LE. This was found to be true regardless of contrast material.
Figure 5. Energy dependence of SC for (a) molybdenum, (b) silver, (c) iodine and (d) gold. SC is only greater than 0 when the LE and HE bracket the k-edge of the material
Download figure:
Standard image High-resolution imageFigure 6. SC of iodine as a function of the HE and LE for energy pairs that bracket the k-edge (33 keV). The data shows that the location of the LE has very little on SC compared to the location of the HE. In addition, the rate of change in SC is greater as the HE approaches the k-edge.
Download figure:
Standard image High-resolution imageThe dependence of CNR on energy and material is demonstrated in figure 7. The graphs are plotted for a breast thickness of 5 cm, with a dose fraction of 0.5 to the LE image. Similar to SC, a large value of CNR is only obtained for energy pairs that bracket the k-edge of the material. As expected, CNR is not observed for materials such as Au whose k-edge lies outside of the energy range. However, the variation in CNR with material is less than that of SC. For example the maximum SC for Ag is 1.6 times that of I, whereas the maximum CNR of Ag is 1.04 times that of iodine at a dose fraction of 0.5. This discrepancy between SC and CNR is a result of the energy dependence of the image noise. The noise in the DE image increases substantially for lower values of either LE and HE because of the poor penetration through the breast, resulting in fewer x-rays being detected. This penalizes materials with lower atomic number because the optimal energies are lower.
Figure 7. Energy dependence of CNR for (a) molybdenum, (b) silver, (c) iodine and (d) gold for a dose distribution of 50% to LE. Similar to SC, CNR is only observed for energy pairs that bracket the k-edge of the material. The choice of contrast material does not have as large an impact on the maximum achievable CNR as it does on SC. The CNR for Au is, however, much lower than the other materials at this dose distribution.
Download figure:
Standard image High-resolution image3.2. Maximum CNR for each material
The maximum CNR for every material in the range of Z = 1 to 80 is shown in figure 8. The corresponding dose fractions that result in these optimal points are overlaid. The graph can be divided into two main regions: (R1) atomic numbers less than 33 or greater than 63, and (R2) atomic numbers between 34 and 62. The materials in R1 exhibit very little contrast due to the absence of a k-edge in the energy range studied. The optimal dose fraction in this range has values between 0.1 and 0.5, which minimizes the noise. The more interesting materials are found in R2. The CNR slowly increases and reaches a plateau for materials with atomic numbers between 42 and 63. Within this plateau, the maximum CNR differs by less than 15%. The absolute maximum occurs at Z = 59, praseodymium, with a dose fraction of 0.2 to the LE at an (LE, HE) energy pair of (18, 42). The dose fraction within R2 reaches its maximum value of 0.7 at an atomic number of 34. As the atomic number increases, the optimal dose fraction decreases until it reaches its minimum value of 0.1 at an atomic number of 63.
Figure 8. Overlay of the maximum CNR and corresponding dose distribution for materials with Z ranging from 1 to 80. There exists a group of materials from Z = 42 to 63, where the maximum CNR does not change by more than 15%. This group of materials represent the optimum selection for contrast agents in CEDE breast imaging.
Download figure:
Standard image High-resolution imageThe trend observed for the dose fraction to the LE image within R2 can be explained by analyzing the variation in W. Figure 9 is an overlay of the optimal dose fraction (from figure 8) and the corresponding values of W. The optimal LE for the materials within the plateau of R2 was consistently calculated to be 18 keV. The static location of the optimal LE is a result of the simulation attempting to minimize the noise in the LE image. A LE value of 18 keV represents an optimal point that achieves this goal. The HE was found to be optimized directly above the k-edge of the material. Thus, as the atomic number (and k-edge) increases, the separation between the LE and HE increases resulting in decreasing values of W. As W decreases, the optimization algorithm opts to allocate a greater portion of the total dose to the HE image to reduce the DE noise in the background (equation (12)). Decreasing the dose fraction to the LE image ensures that the noise will reach a minimum value, and thus maximize the CNR.
Figure 9. Overlay of the optimal weighting factor leads to the maximum CNR (from figure 8) and the corresponding dose fractions to the LE.
Download figure:
Standard image High-resolution image3.3. Candidate contrast materials for CEDE breast imaging
Table 1 lists some of the materials that make up the plateau of maximum CNR in figure 8. Listed for each material are the atomic number, k-edge, maximum CNR, optimal dose fraction, optimal energy pair and current nanoparticle-based research applications. As observed from the current nanoparticle-based imaging research, none of these materials, apart from clinically used iodine, have been investigated for application as DE imaging agents. Nanoparticles are particularly beneficial for use as contrast agents due to the ability to finely tune their physical and function characteristics and so directly control their interactions in the body (Wang and Thanou 2010). For example, nano-silver represents the largest (25%) and fastest growing category of nanotechnology-based consumer products (Liu and Hurt 2010). The majority of these applications make use of the broad-spectrum antimicrobial and unique optical scattering properties of silver (Chen et al 2010, Mehra et al 2013, Musarrat et al 2010, Kahraman et al 2010, Nabikhan et al 2010, Verma et al 2010). There has been little interest to date in developing silver nanoparticles as x-ray imaging agents, but the data presented here suggests silver offers comparable CNR to iodinated contrast agents with more achievable energy pairs and appropriate dose fractions. Similarly, molybdenum lies within the plateau of materials in R2. Molybdenum oxide nanoparticles have been utilized as hosts for ion insertion with applications as catalysts, gas sensors, fuel cell membranes, electrochromic windows and lithium-ion batteries (Lee et al 2009). However, Mo nanoparticles have yet to be investigated as potential CEDE imaging agents.
Table 1. Potential contrast materials for DE breast radiography. The materials are listed with their atomic number, maximum SC (DE contrast), improvement over iodine and current nanoparticle-based research. The improvement over iodine is calculated as the as ratio of the maximum SC of the contrast material to that of iodine. Current nanoparticle-based research applications for each material are also listed.
| Material | Z | K-edge | Maximum CNR | Optimal dose fraction to LE | Optimal energy pair (LE,HE) | Current nanoparticle-based research applications |
|---|---|---|---|---|---|---|
| Molybdenum | 42 | 20.0 | 40.3 | 0.46 | (18,23) | Catalysts, fuel cell membranes (Lee et al 2009) |
| Rhodium | 45 | 23.2 | 39.0 | 0.40 | (18,26) | Catalysis (Lee et al 2005) |
| Palladium | 46 | 24.4 | 40.4 | 0.40 | (18,26) | asymmetric catalysis (Yoon and Wai 2005) |
| Silver | 47 | 25.5 | 42.6 | 0.40 | (18,26) | Antimicrobial, optical scattering (Nabikhan et al 2010, Verma et al 2010, Musarrat et al 2010, Mehra et al 2013, Kahraman et al 2010, Chen et al 2010) |
| Tin | 50 | 29.2 | 44.1 | 0.33 | (18,30) | Gas microsensors, Li-ion batteries (Wang et al 2004) |
| Tellurium | 52 | 31.8 | 41.9 | 0.34 | (18,32) | Semiconductor in electronic and opticalelectronic (Xi et al 2005) |
| Iodine | 53 | 33.2 | 42.6 | 0.32 | (18,34) | X-ray contrast agents Zalutsky et al 1987) |
| Barium | 56 | 37.4 | 44.1 | 0.25 | (18,38) | Capacitors, non-linear optics (Nuraje et al 2006) |
4. Discussion
CEDE breast x-ray imaging represents an exciting new imaging tool in the detection and characterization of breast lesions (Chen et al 2008, Carton et al 2010a, 2010b, 2010c, Dromain et al 2012, Froeling et al 2013, Jochelson et al 2013). The technique suppresses the variation in anatomical signal intensity to improve the detection and quantification of exogenous imaging agents. Currently, CEDE imaging is performed with iodinated contrast agents to visualize tumor vasculature. These agents are, however, prone to certain deficiencies such as off-target accumulation, toxicity and low circulation times. Our work is intended to identify novel contrast materials whose contrast is comparable or superior to that of iodine in the mammographic energy range so that an agent optimized for DE breast imaging may be discovered.
The DE signal intensity is formulated as the weighted logarithmic subtraction between the LE and HE images. The signal intensities of the LE and HE images are expressed in terms of the linear attenuation coefficients and thicknesses of the adipose, glandular and contrast material. The resulting weighting factor and CNR can be explicitly formulated using these factors. The formulation is similar to the subtraction weighting factor, R, and DE signal-to-noise ratio described by Brettle et al (Brettle and Cowen 1994). There are, however, several important distinctions. Brettle's analysis was geared towards the isolation of microcalcifications from the soft-tissue background using DE mammography. In addition, their analysis combined the attenuation of the adipose and glandular tissue into a singular compartment, called breast tissue. On the other hand, our analysis is focused on the visualization of an external contrast agent using k-edge imaging. The breast background is broken down into adipose and glandular tissue, and the weighting factor is calculated to remove the signal variation that arises from various admixtures of these tissue components.
In the calculation of W, the total breast thickness under compression is assumed to be constant across the image. This has several practical consequences. The amount of compression applied during the DE examination may need to be increased to ensure that the variation in thickness across the breast is minimal. However, increasing the compression force is likely to alter the blood kinetic profiles of the contrast agent and restrict the delivery of the contrast agent to the breast as an organ. Alternatively, a thickness-dependent weighting factor can be used to subtract regions towards the edge of the breast that may be otherwise poorly subtracted due to the uneven thickness of tissue. It is important to note that W, as calculated in this monoenergetic model, is independent of the thickness of the breast. This is not true for polyenergetic spectra where secondary effects such as beam hardening can alter the effective attenuation coefficients of materials depending on thickness. Thicker materials will appear to have lower effective attenuation coefficients than their thinner counterparts.
This study also ignores the influence of scatter. Scattered x-ray photons can give the appearance of a lower thickness of tissue in the beam path. Thus, as long as the scatter is constant across the breast, the proposed subtraction technique is able to remove the anatomical signal variation effectively. Constant scatter would simply add an offset in equation (9a) and would therefore not affect the calculation of W. Scatter does, however, play an important role in the calculation of the maximum CNR for various materials. The fraction of primary x-ray photons that are scattered is lower at lower energies, and would therefore not be constant across the various materials. As previously mentioned, the framework is built upon the assumption of a monoenergetic x-ray source. The translation of this work into polyenergetic x-ray beams would require some modification to the framework to account for the variation in attenuation coefficients of adipose, glandular and contrast material with energy. The findings presented here can serve as a reference for a more detailed analytical comparison of contrast materials using polyenergetic spectra.
The analysis identified a group of materials from atomic numbers 42 to 63 that maximize CNR in DE breast imaging. As the atomic number increases, the HE value increases, and the dose fraction decreases. Iodine, with Z = 53, lies within the midpoint of these materials. The optimal imaging protocol for iodine requires an energy pair of (LE, HE = 18, 34) and a dose fraction of 0.32 to LE. In a practical sense, high mean energies, such as 34–49 keV, require high kV together with substantial filtration to achieve a suitable HE spectrum (Wu and Liu 2004). The resulting reduction in photon fluence requires the tube current-time product (mAs) to be increased to accommodate for the reduced fluence. Clinical acquisition systems have limitations on tube loading and exposure time which may prevent optimal high-energy spectra being achieved for high Z materials. In addition, maximizing CNR for high Z materials requires that a low dose fraction to the LE image be used. Low dose LE images will be intrinsically noisy and will limit the anatomical information that can be provided by DE breast imaging.
Following this logic, lower Z materials from the plateau region may be more favorable based upon more achievable energy pairs, and dose fractions that provide good quality anatomic images. As iodine is currently the standard in CE breast imaging, despite possessing several limitations, it can be treated as the highest feasible atomic number contrast agent. Therefore, contrast agents with atomic numbers between 42 and 52 should be superior for CEDE breast imaging. These materials need to be further investigated in terms of toxicity, delivery mechanism and ease of fabrication to identify novel contrast agents specifically designed for DE imaging in the breast.
5. Conclusions
This work identifies novel contrast materials that are potentially superior to iodine for CEDE x-ray breast imaging. Iodinated agents display excellent stability in the body while resisting degradation over long periods of time. However, they are plagued by rapid clearance, poor tumor targeting ability and contrast media-induced toxicity. Through analytical modeling, a group of materials with atomic numbers ranging from 42 to 63 was identified to maximize CNR values in the mammographic energy range. Several of these materials have been investigated in nanoparticle-based applications but none, other than iodine, have been investigated for applicability as imaging agents in CEDE x-ray breast imaging. Of these materials, those with lower Z, between 42 and 52, may be more favorable by offering more achievable energy pairs and appropriate LE dose fractions. These results represent the first step in the development of new contrast agents optimized for DE x-ray breast imaging.
Acknowledgment
This work was supported in part by the US Department of Defense W81XWH-09-01-0055.










