Abstract
Use of 15O labeled oxygen (15O2) and positron emission tomography (PET) allows quantitative assessment of the regional metabolic rate of oxygen (CMRO2) in vivo, which is essential to understanding the pathological status of patients with cerebral vascular and neurological disorders. The method has, however, been challenging, when a 3D PET scanner is employed, largely attributed to the presence of gaseous radioactivity in the trachea and the inhalation system, which results in a large amount of scatter and random events in the PET assessment. The present study was intended to evaluate the adequacy of using a recently available commercial 3D PET scanner in the assessment of regional cerebral radioactivity distribution during an inhalation of 15O2. Systematic experiments were carried out on a brain phantom. Experiments were also performed on a healthy volunteer following a recently developed protocol for simultaneous assessment of CMRO2 and cerebral blood flow, which involves sequential administration of 15O2 and C15O2. A particular intention was to evaluate the adequacy of the scatter-correction procedures. The phantom experiment demonstrated that errors were within 3% at the practically maximum radioactivity in the face mask, with the greatest radioactivity in the lung. The volunteer experiment demonstrated that the counting rate was at peak during the 15O gas inhalation period, within a verified range. Tomographic images represented good quality over the entire FOV, including the lower part of the cerebral structures and the carotid artery regions. The scatter-correction procedures appeared to be important, particularly in the process to compensate for the scatter originating outside the FOV. Reconstructed images dramatically changed if the correction was carried out using inappropriate procedures. This study demonstrated that accurate reconstruction could be obtained when the scatter compensation was appropriately carried out. This study also suggested the feasibility of using a state-of-the-art 3D PET scanner in the quantitative PET imaging during inhalation of 15O labeled oxygen.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
For more information on this article, see medicalphysicsweb.org
1. Introduction
The use of 15O-labeled molecular oxygen (15O2) and positron emission tomography (PET) provides the unique opportunity of using tomographic imaging of quantitative regional metabolic rate of oxygen in clinical settings. The technique has contributed to a number of clinical studies to understand the pathophysiological status of cerebral vascular and other disorders, as reviewed in a recent article (Baron and Jones, 2012). These measurements are achieved by PET scanning while 15O-labeled oxygen is inhaled, either during a continuous ( Lammertsma et al 1981 Jones et al 1982, Lammertsma et al 1982) or following a short-period supply (Mintun et al 1984, Ohta et al 1992, Kudomi et al 2005, Kudomi et al 2013) of 15O-labeled oxygen in gaseous form.
Of the note is that the entire radioactivity should be supplied through the trachea and the inhalation system, which are mostly inside the field-of-view (FOV) of PET imaging, and can cause increased scattered and random events. There is also strong radioactivity in the lung region during the inhalation, which also causes increased random events. Such factors have not been considered problematic when traditional PET devices were utilized in 2D mode, because PET detectors were well shielded from annihilation photons emitted outside of the tomographic slices by the slice-separating shield or Septa. This is, however, not the case in 3D PET, because the strong radioactivity in the trachea and the inhalation system increased single events and thus increased random events in proportion to the square of the amount of radioactivity. The dead time count loss can then occur at increased random rates. Ibaraki et al (Ibaraki et al 2011) demonstrated that a shielding device was effective in reducing the random events during the 15O-oxygen inhalation, improving the noise-equivalent count rate (NECR). The radioactivity in the trachea area and the inhalation system can also increase the scatter. To avoid the errors attributed to these factors, many of the 15O-oxygen PET scans have been carried out using a 2D PET mode even in recent studies (Nezu et al 2012, Kaku et al 2013, Kawai et al 2014, Kudomi et al 2013).
Recently, 3D acquisition became standard and 2D PET acquisition is no longer supported in commercial products, attributed to the advantage of increased sensitivity for oncological studies using 18F-fluorodeoxiglucose (FDG). It is also important to note that the physical performance of the recent 3D PET devices has been dramatically improved in both the hardware and software and potentially provides quantitatively accurate images. Significant improvements are in the performance of the detector block assembly for accurate and precise positioning and energy identification of each photon signal (Melcher and Schweitzer 1992), faster electronics (Puterbaugh et al 2004) for a shorter coincidence time window, faster logic operation, data transfer and storage. These resulted in improved counting-rate performance in terms of the reduced random events, increased NECR, reduced dead-time count losses, better count-rate independent accuracy and uniformity, etc. The attenuation map-based scatter correction (Ollinger 1996, Watson et al 1996, Watson et al 1997,Watson 2000), the pre-smoothing operation of random events (Brasse et al 2005, Panin 2008) and the implementation of the position-dependent point-spread function (Panin et al 2006), as well as the time-of-flight information ( Surti et al 2007, Karp et al 2008, Lois et al 2010) into the reconstruction are considered to have improved the image quality and accuracy in the 3D PET acquisition. All these improvements are essential in supporting the adequacy of applying the new-generation reconstruction algorithms to the scatter correction by means of the 'single-scatter simulation (SSS)' method (Ollinger 1996, Watson et al 1996, Watson et al 1997, Watson 2000). This SSS method stands for the Klein-Nishina formulation to describe a relationship between the energy loss and angular distribution. The method was recently extended to compensate for the scatter originating outside-FOV (Watson et al 2004), in which the lines-of-response (LORs) that indicated small attenuation were omitted from the reconstruction, and the scatter inside the object was estimated from such information. This technique has now been utilized in most major products of clinical PET devices. The 15O-oxygen inhalation PET still requires careful evaluation of such a technique because a large amount of radioactivity exists in gaseous form inside the FOV. This radioactivity exists in the face mask, which has very low attenuation, and thus causes a high rate of true events. The new-generation scatter-correction software attempts to refine the estimate of scatter by using the scatter in the background (outside the attenuation) as a reference for the simulated scatter. Since the face mask has very low attenuation, the scatter algorithm can confuse the true events due to the activity in the facemask with scattered events in the background. In addition, increased amounts of random and scattered events are to be introduced by the radioactivity in the 15O-oxygen supply system and also in the lung. These factors could degrade the image accuracy and quality and thus the adequacy of the reconstruction procedures need to be evaluated carefully.
The present study was intended to evaluate the adequacy of using the state-of-the-art 3D PET scanner in the quantitative PET examination with the inhalation of 15O-labeled oxygen. We particularly aimed at evaluating the physical accuracy of the regional cerebral radioactivity assessment under a realistic condition, in the presence of gaseous 15O-oxyen in the face mask attached to the object. Adequacy of the scatter-correction method was also tested, for settings both with and without the compensation for the outside-FOV scatter. We also examined effects of the presence of strong radioactivity in the lung area on the accuracy of cerebral radioactivity quantitation. The systematic evaluation was carried out using a recently developed 3D brain phantom, and also in a healthy volunteer, during the inhalation of 15O-labeld gaseous oxygen.
2. Materials and methods
2.1. PET scan procedures
The PET scanner used was Biograph-mCT (Siemens, Knoxville, USA), a hybrid CT/PET tomograph. Specification and physical performance of this scanner was reported in a recent article (Jakoby et al 2011). Briefly, the scanner provided tomographic PET images for 109 slices for an in-plane FOV diameter of 700 mm and an axial FOV of 218 mm. The spatial resolution was 4.4 mm in full width at half maximum (FWHM) at the center of FOV. The coincidence time window was 4.1 ns. The maximum NECR of approximately 180 kcps was obtained with smoothed random correction technique (Panin 2008) at the radioactivity concentration of approximately 28 kBq ml−1, using a NEMA scatter phantom.
A series of PET scans were carried out in a list mode on a 3D brain phantom, which simulated a static cerebral blood-flow distribution in the grey-matter regions, with inclusion of the skull structure and a realistic head contour (Iida et al 2013). The grey-matter component of this phantom was filled with 18F solution of 10 MBq, which is equivalent to the radioactivity in the brain in typical clinical scans following inhalation of 15O-oxygen of approximately 400 MBq. A clinical face mask was attached to this phantom, to which a radioactive gas containing 15O-oxygen was supplied. This was intended to mimic the possible degradation of image quality due to the high gaseous radioactivity surrounding the patient head. An additional cylindrical phantom filled with 18F of 80 MBq was prepared, in order to simulate the contribution of high radioactivity in the lung during the 15O-oxygen inhalation. This radioactivity in this cylindrical phantom was equivalent to that in the entire lung at the peak during the 15O-oxygen inhalation.
Following a low-dose x-ray CT acquisition for attenuation coefficient map, the experiment was carried out for 4 different conditions, as illustrated in figure 1. In the first case (case 1), the scan was carried out on the 3D brain phantom alone, which was assigned as a reference to the following experiments. Then afterward, as the second case (case 2), radioactive gas containing 15O-labeled oxygen was supplied continuously to the face mask during the PET scanning. The gaseous radioactivity inside the face mask was changed as 0.2, 0.7, 2.0, 4.6 and 18.4 MBq and the maximum radioactivity was approximately twice the peak radioactivity in the face mask in typical clinical scans. In this experiment, we intended to place the entire face mask containing the majority of gaseous radioactivity inside the FOV, except for a part of gas-supplying tubes. As the third case (case 3), the PET scan was performed as an extension of case 2, but by placing an additional cylindrical phantom containing 80 MBq on the bed, at a distance of 15 cm from the brain phantom. The scan was then carried out, similarly to the case 2, by changing the radioactivity up to 18.4 MBq. As the fourth condition (case 4), the scan was performed similarly to case 2, but by moving the bed position towards the feet direction at 7.5 cm. The radioactivity was changed up to 4.6 MBq in this experiment. The majority of gaseous radioactivity in the face mask of axial length of approximately 10.5 cm was mostly outside of FOV. In all cases, data was acquired in list mode for 3 min at each case and was sorted to generate a dynamic sinogram at every 30 s, including corrections for detector normalization, random coincidence and dead time count losses.
Figure 1. Four configurations in the experiment performed on the 3D phantom. In the first case, the scan was carried out on the 3D brain phantom alone, filled with 18F-solution into the grey-matter component. Then afterward, as the 2nd case, radioactive gas containing 15O-labeled oxygen molecules was supplied continuously to the face mask during the PET scanning. As the 3rd case, the PET scan was performed as an extension of case 2, but by placing an additional cylindrical phantom containing 80 MBq on the bed, which corresponded to the maximum radioactivity in a typical clinical study during the inhalation of 15O2. In the 4th case, the scan was performed similarly to case 2, but by moving the bed position towards the feet direction at 7.5 cm. The majority of gaseous radioactivity contained in the face mask of axial length of approximately 10.5 cm was outside of FOV. In all cases, PET scans were initiated during the continuous supply of gaseous radioactivity of 0.2, 0.7, 2.0, 4.6 and 18.4 MBq inside the face mask, except for case 4, in which the gaseous radioactivity was supplied up to 4.6 MBq.
Download figure:
Standard image High-resolution imageAnother PET scan was carried out on a human subject, according to a recently proposed protocol of Kudomi et al (Kudomi et al 2013). The subject was a male volunteer of 55 years old, who had no signs or symptoms of cerebral vascular diseases, as confirmed by MRI. The subject was positioned on supine with the specially designed face mask, so as to ventilate inside the facemask while supplying the radioactive gases. Special attention was paid to placing the facemask inside the FOV to confine the majority of the gaseous radioactivity inside of FOV. Following a low-dose x-ray CT acquisition for imaging the attenuation coefficient map, an 8 min PET scan was carried out in list mode during the sequential inhalation of 15O-oxygen (1,600 MBq in 1 min) and 15O-carbon dioxide (1,600 MBq in 1 min) and was sorted to generate dynamic sinogram. Corrections were carried out for detector normalization, random coincidence and dead-time count losses. The study was approved by the ethics committee of the National Cerebral and Cardiovascular Center, Osaka, Japan. A written informed consent was obtained.
2.2. Data processing
Dynamic tomographic images were reconstructed using the 2D filtered-back-projection (FBP) algorithm from the re-binned sinogram, obtained using the Fourier Rebining (Defrise et al 1997). The matrix size on the reconstructed images was 128 × 128 × 75 for both phantom and human studies. The voxel size was 2.55 × 2.55 × 3.00 mm3 and 2.27 × 2.27 × 3.00 mm3, corresponding with the phantom and human experiments, respectively. The dynamic images consisted of 6 frames of 30 s for phantom reconstructions and 6 frames of 10 s, 6 frames of 20 s, 4 frames of 30 s, 12 frames of 5 s, 9 frames of 10 s and 2 frames of 15 s for the volunteer study. Corrections were implemented in the reconstruction process for the detector normalization, the random coincidence, the dead time, the radioactive decay, the attenuation and the scatter. A Gaussian post filter of 6 mm FWHM was applied to the reconstructed images.
The scatter correction was carried out using the single scatter simulation technique (SSS) of Watson et al (Watson 2000, Watson et al 1996, Watson et al 1997). The technique simulates the scattered sinogram from the emission sinogram using the x-ray CT-based attenuation map, by means of the Klein-Nishina formulation. In this study, the scatter correction was made in two ways: (a) to include the process estimating the scatter distribution originated from outside-FOV, namely to scale the estimated scatter sinogram by referring LORs that indicated a small attenuation factor with a threshold level of a small attenuation factor (Watson et al 2004) and (b) to disable the process to compensate for the scatter originated from outside of FOV, namely to utilize the estimated scatter sinogram as is (no scaling). Neither point-spread function (PSF) reconstruction (Panin et al 2006), nor time-of-flight (TOF) reconstruction (Watson 2007) were carried out in this study.
2.3. Data analysis
Reconstructed images were transferred to a PC workstation (Mouse computer Ltd, Kasukabe City, Japan) and further analysis was carried out on this computer. The dynamic images had been already corrected for the radioactive decay of 15O (a half-life of 122.24 s), back to the PET scan start time. In this study, the decay was added to all images for each frame, so that pixel counts of each image corresponded to the momentary radioactivity concentration at the time of imaging. Four regions-of-interest (ROIs) were drawn manually on the cerebellum, the temporal lobe, the basal ganglia and the parietal cortex areas, on images obtained from the 3D phantom and from volunteer experiments, using a QView software developed in this institute (http://www.qspect.org) (Iida et al 2010) (figure 2).
Figure 2. A comparison of the 3-dimensional brain phantom images: (a) attenuation coefficient images estimated from the x-ray CT scan, (b) the design images of the grey-matter component smoothed with a Gaussian filter with 9.0 mm FWHM, which corresponds to the spatial resolution of PET images, (c) PET images from the case 1 experiment, (d) PET images from the case 2 experiment with the scatter correction, including the outside-FOV compensation and (e) the same as (d), but with the outside-FOV scatter compensation disabled. Arrows a indicate the radioactivity in the face mask and arrows b the radioactivity of the 15O2-supplying tube.
Download figure:
Standard image High-resolution imageIn the phantom experiment, errors were assessed for each of the 3 experimental conditions (cases 2–4), as difference from the images were measured in the case 1 experiment. Mean and one standard deviation values were calculated from the 6 frame images, on which 4 ROIs were selected on the cerebellum, frontal part of the temporal lobe, basal ganglia and parietal lobe regions. The mean errors were then plotted as a function of the total radioactivity in the facemask for the two scatter-correction procedures – i.e., one with the inclusion of outside-FOV scatter-correction procedures and another without the inclusion, as noted in the data-processing section. The prompts, random and true (+ scatter) coincidence events rates and consequently the NECR were plotted as a function of the total radioactivity in the face mask for each of the 3 experimental conditions of cases 2, 3 and 4.
In the human study, two images were calculated by integrating over the initial 300 s after the initiation of 15O2 and 150s after C15O2 inhalation, from which maximum intensity projection (MIP) images were generated. These images correspond to the relative distribution of the cerebral metabolic rate of oxygen (CMRO2) and the cerebral blood flow (CBF), respectively. Differences between the two scatter-correction procedures were then visually evaluated in these integrated images. Prompt, random and true coincidence rates and NECR were also plotted as a function of time for the two scatter-correction techniques. Regional radioactivity concentrations in units of kBq ml−1 were also obtained from the dynamic PET images for the 4 ROIs, from which differences between the 2 scatter-correction procedures were evaluated. Results were presented as mean ± 1 standard deviation (s.d.).
3. Results
Figure 2 shows results from the 3-dimensional brain phantom experiments. Images of the phantom filled with 18F solution, shown in the row c, were in agreement with the design images after smoothing with the Gaussian filter shown in row b. Relative counts in each region differed less than 2% in all areas of the phantom (Iida et al 2013). Strong gaseous radioactivity was visible in the face mask and the waste tube in the case 2 experiment, as shown in rows d and e. It was also notable that the radioactivity was underestimated in the cerebellum, the lower part of the temporal lobe and the frontal regions when the scatter correction was carried out, including the compensation occurring outside FOV (row d). Despite the presence of streaking artifacts caused by the strong radioactivity in the face mask, the regional radioactivity distribution was recovered by choosing the original scatter-correction technique (Watson 2000), which did not account for the outside FOV scatter (row e) and became closer to the reference images obtained from the case 1 experiment (the row c) as well as to the smoothed design of the grey-matter structure (row b). No clear difference was visible in the parietal and basal ganglia regions between the two scatter-correction procedures, with and without accounting for the outside-FOV scatter.
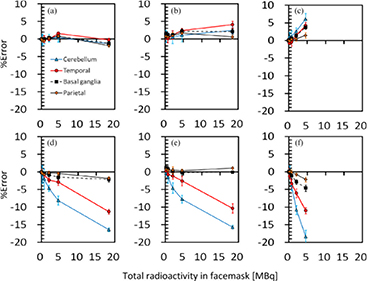
Figure 3 also shows results from the 3D brain phantom experiments, which demonstrate the magnitude of errors in the regional radioactivity assessment for the given ROIs as a function of radioactivity in the face mask for each of cases 2‒4, shown in figure 1. When the radioactivity was supplied only to the face mask (case 2), the difference from the brain phantom alone (case 1) was less than +/ − 2% in all regions, when the outside-FOV scatter correction procedures were excluded. However, when the outside-FOV scatter compensation procedures were included, errors were increased in all regions and reached −10% and −15% in the temporal lobe and cerebellum regions, respectively. For case 3, in which additional radioactivity was placed in the lung area, which corresponded to the maximum dose during inhalation of 15O2 in a clinical study, errors were slightly increased to 3% at 10 MBq of gaseous radioactivity in the face mask, when the outside-FOV scatter compensation was excluded. Inclusion of the outside-FOV scatter correction increased the errors to a similar level to those in case 2, but they were consistently worse than those without the outside-FOV scatter correction. When part of the face mask was placed outside FOV, errors were 2‒6%, and −2‒19% at the radioactivity of 5 MBq inside the face mask, corresponding to the exclusion and inclusion of the outside-FOV scatter correction, respectively.
Figure 3. Errors in the regional radioactivity concentration for the 4 ROIs selected on the reconstructed PET images as a function of radioactivity in the face mask for three different configurations of case 2 (a, d), case 3 (b, e) and case 4 (c, f) shown in figure 1. All data was corrected for the scatter by means of the single-scatter simulation method, with and without the inclusion of the outside FOV scatter, corresponding to (a, b, c) and (d, e, f), respectively. Blue solid, red solid, black dashed and black solid lines correspond to the cerebellum, temporal lobe, Basal ganglia and Parietal regions, respectively.
Download figure:
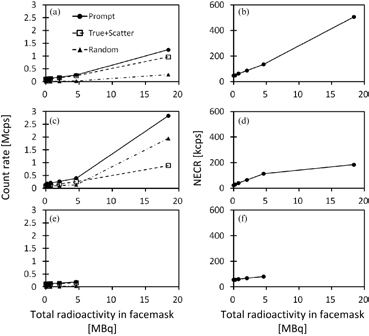
Standard image High-resolution imageFigure 4 shows counting-rate characteristics observed in the 3D brain-phantom experiment for each of cases 2, 3 and 4, namely the prompt, true + scatter and random events in the left column, as well as NECR in the right column, as a function of radioactivity inside the face mask. For the case 2 experiment, true rates increased when radioactivity in the face mask was increased almost linearly, as the random rates were small. NECR also increased almost linearly as the face-mask radioactivity was increased. In case 3, on the other hand, random rates were considerably increased compared with case 2. The random rates were even higher than the true + random rates, with the total radioactivity in the face mask being greater than 5 MBq. NECR in case 3 (figure 4(d)) tended to be saturated at the face mask radioactivity >5 MBq, and smaller than in case 1 (figure 4(b)). In case 4, the experiment was carried out only for a range of the face-mask radioactivity <5 MBq. The NECR shown in figure 4(f) reflect smaller values than in case 2 at the total radioactivity in the facemask <5 MBq.
Figure 4. Counting-rate characteristics as a function of radioactivity in the face mask observed in the 3D brain-phantom experiments for three different configurations of case 2 (a, d), case 3 (b, e) and case 4 (c, f), shown in figure 1. Figures a, c and e represent prompt, true + scatter, and random count rates for the three configurations and figure b, d, and f represent NECR.
Download figure:
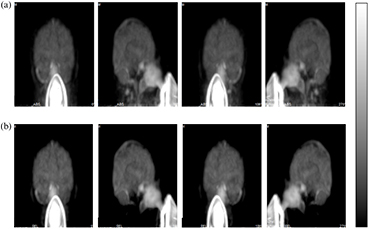
Standard image High-resolution imageFigure 5 shows comparison of the MIP calculated from PET images integrated over the initial 300 s after the start of the 60 s 15O2 inhalation, which were obtained from a healthy volunteer study. The two sets of images were with the scatter-correction procedures (a) that did not involve the outside-FOV scatter compensation and (b) that involved the outside-FOV scatter compensation. Regional radioactivity distribution is reasonable over the entire FOV when the outside-FOV scatter compensation was not involved. However, when the outside-FOV scatter correction procedures were applied to the reconstruction process, the carotid arterial regions were not visible. Also, part of the cerebellum and temporal lobe areas were missing. This is similar to the findings in the phantom experiments shown in figure 2. No clear differences were seen between the two procedures in the upper part of the cerebral regions.
Figure 5. Maximum intensity projection (MIP) images obtained from a healthy volunteer study during 15O2 inhalation. Image reconstruction involved the scatter correction with two different procedures. The upper row (a) represents images with the scatter correction, excluding outside-FOV scatter-compensation procedures and the lower row (b) represents images with the outside-FOV scatter compensation procedures.
Download figure:
Standard image High-resolution imageFigure 6 shows the count-rate characteristics observed in the healthy volunteer study with the sequential inhalation of 15O2 and C15O2. Prompt, true + scatter and random rates shown in figure 6(a) demonstrated that both increased during the 15O2 and C15O2 inhalation periods, with smaller magnitude during the 15O2 than C15O2 inhalation period. The peak count rate for the random was approximately 0.8 Mcps and 1.4 Mcps, with the random-to-true + scatter ratio of approximately 2.5 and 2.0 during the 15O2 inhalation and C15O2 inhalation periods, respectively. The random rate relative to the true + scatter appeared to be greater during the 15O2 inhalation than during the C15O2 inhalation period, and the ratio was also greater during the 15O2 inhalation period than C15O2. NECR shown in figure 6(b) also demonstrated smaller values during the 15O2 inhalation period than C15O2. Figure 6(c‒f) showed regional time-activity curves (TTAC) obtained from the reconstructed PET images for the 4 ROIs on the cerebellum, emporal lobe, basal ganglia, and parietal lobe regions. TTAC showed reasonable shape when the outside-FOV scatter compensation was not applied. Inclusion of the outside-FOV scatter compensation procedures resulted in null counts at the beginning in TTACs in the cerebellum and temporal lobe regions.
Figure 6. Counting-rate performance observed in the volunteer experiments as a function of time obtained with the sequential inhalation protocol of 15O2 and C15O2. (a): prompt, true + scatter, and random coincidence rates for the whole FOV; (b): NECR; (c): regional time-activity curves in the cerebellum region, when images are reconstructed with the scatter correction and implementing the outside-FOV scatter compensation; (d): the same as c but in the temporal lobe region; (e): the same as c and d but in the region of the basal ganglia and (f): the same as c, d, and e but in the region of parietal lobe.
Download figure:
Standard image High-resolution imageFigure 7 shows the difference in the two scatter-compensation algorithms. Figures 7(a) and (b) show the emission sinogram and the attenuation correction derived from the CT image. Figure 7(c) shows the area used for scaling if scaling is included. Figures 7(d) and 7(e) show the performance of the scatter-scaling algorithm. Figure 7(d) shows the algorithm that does not scale external scatter, while figure 7(e) shows the algorithm without FOV scaling. In each plot, the red line (total emission) shows the measured sinogram, while the green line (scatter) shows the simulated and scaled scatter. The blue lines show the regions where the two are scaled. In figure7(e), the scatter is obviously over-scaled, causing underestimation of the emission activity.
Figure 7. The emission sinogram (figure 7(a)) and attenuation correction derived from the CT image (figure 7(b)). The region of the sinogram where scaling for out-of-FOV scatter is done (figure 7(c)). The profile through the sinogram showing the emission and simulated scatter when scaling is not applied (figure 7(d)). The profile through the sinogram when out-of-FOV scaling is applied (figure 7(e)).
Download figure:
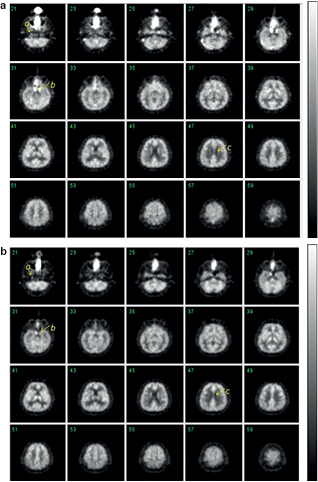
Standard image High-resolution imageFigure 8 showed the integrated transaxial PET images obtained from the healthy volunteer with the sequential inhalation protocol of 15O2 and C15O. Detailed structure of cortical grey-matter regions are reasonably visible in both relative CMRO2 and CBF images, as shown in figures 8(a) and (b), respectively. Identification of the carotid arterial lines, as well as the separation of the carotid sinus from the temporal lobe, is also well recognized. Separation of the white matter area from the cerebral spinal fluid space and the grey matter area can also be seen in these images.
Figure 8. Transaxial PET images obtained from the healthy volunteer integrated over the initial 300 s after the 15O2 inhalation (figure 8(a)) and the initial 150 s after the C15O2 inhalation (figure 8(b)). The protocol essentially followed the recently proposed dual-administration protocol on the human volunteer (Kudomi et al 2013). Arrow a indicates one of the carotid arterial lines, arrow b represents the sinus demonstrating a reasonable separation that has not been done well using previous PET scanners and arrow c demonstrates a good separation of white matter area from cerebral fluid space and cortical grey-matter regions.
Download figure:
Standard image High-resolution image4. Discussion
This study demonstrated that the state-of-the-art 3D PET scanner can provide accurate assessment of cerebral radioactivity distribution during the inhalation of gaseous 15O-labeled oxygen and 15O-labeled carbon dioxide. Recently developed advanced technologies, include hardware such as sophisticated detector block units (Melcher and Schweitzer 1992), electronic devices (Puterbaugh et al 2004) and software such as the pre-smoothing method (Brasse et al 2005), appropriate conversion of CT images to the attenuation mu distribution (Carney et al 2006), enabled high spatial resolution, NECR and signal-to-noise ratio. All of these features supported the adequacy of utilizing scatter-correction procedures that applied the SSS algorithm of Watson (Watson 2000). Although not employed in this study, the reconstruction, including the point-spread function (PSF) model (Panin et al 2006) and the time-of-flight (TOF) reconstruction algorithm (Surti et al 2007, Karp et al 2008, Lois et al 2010), might contribute to improving spatial resolution as well as image quality, as has been demonstrated in whole-body PET scanning (Schaefferkoetter et al 2013).
The scatter correction algorithm described by Watson (Watson et al 2004) first simulates the scatter using the emission image and the transmission or mu image. However, the algorithm is blind to any emission or transmission outside the field-of-view and thus can only simulate the scatter from the emission and transmission data in the field-of-view. Since emission events outside the FOV can scatter into the FOV, Watson (Watson et al 2004) describes an additional compensation to attempt to correct these events. This compensation simply derives a scale factor for the simulated scatter events outside the region of attenuation by comparing it to the measured events in the same region. This procedure to compensate for the out-of-FOV scatter appeared to be inadequate in the 15O-oxygen inhalation study and gave inaccurate reconstruction in the lower part of the brain and as well as the carotid artery regions. This is attributed to the fact that the gaseous radioactivity distributing outside of the head but inside the face mask was mistaken for scatter by the scaling algorithm due to the small attenuation factors of the mask. The scaling algorithm thus scales the simulated scatter to match the radioactivity in the mask, resulting in over-scaling. In this phantom study, when the outside-FOV scatter compensation procedure was disabled, reasonable reconstructed images resulted, even though high radioactivity existed in the face mask. Since the axial FOV of 21.8 cm can cover most of radioactivity in the head as well as the facemask radioactivity, the elimination of the outside FOV scatter is reasonable in terms of predicting the scatter distribution over the entire FOV. However, as has been demonstrated by the phantom experiments in this study (case 4 in figure 1), the presence of the radioactivity outside FOV can cause large errors. It is important to make sure that majority of the radioactivity is covered in the FOV if one intends to maximize the accuracy of the SSS-based scatter correction.
The normal volunteer data demonstrated that the random rates were higher than the true + scatter event rates during the inhalation period both for 15O2 and C15O2. This is consistent with the results from the phantom experiment. When strong radioactivity was placed in the lung region, corresponding to the peak radioactivity in the clinical scans during the 15O2 inhalation, the random rates were much higher than the true + scatter rates, while the reverse was true when no radioactivity was placed in the lung area. It should be noted that there was systematic offset in the regional radioactivity concentration when gaseous radioactivity was supplied (figure 3(b)). The exact reason is not clear, but it is likely attributed to the accidental coincidence of the single events originating from the lung radioactivity and the scattered single photons originally emitted from the gaseous radioactivity. However, the magnitude is small and may not cause major impact to clinical studies in practice. It should also be noted that the pile-up of the nuclear signals is unlikely to happen under the count rate of the present study. The dead-time count loss is also found to be small. These are the advantages of using the PET scanner with improved electronics employed in this study.
The same amount of radioactivity was inhaled in the volunteer study for the 15O2 and C15O2 scans, but the regional tissue radioactivity was approximately 4 times higher during the C15O2 inhalation than during the 15O2. This is partly attributed to the greater amount of extraction into the blood from the air in the lung of C15O2 than 15O2, caused by the carbonic anhydrase and also partly due to the greater extraction of H215O than 15O2 in the brain. Consequently, statistical noise in the integrated images was slightly better for C15O2 than for 15O2. Optimization of the administration dose is warranted, but this is beyond the scope of this study. Due to the non-linear nature between the original PET and functional images, careful analysis is needed.
In this study, we applied a standard FBP reconstruction to the 2D sinogram generated by the Fourier re-binning procedures of Defrise et al (Defrise et al 1997). Recently the point-spread function (PSF)-based reconstruction of Panin (Panin et al 2006) and/or time-of-flight (TOF) reconstruction were shown to improve image accuracy and statistical noise properties (Karp et al 2008, Lois et al 2010). Radioactivity distribution in the volunteer study demonstrated that the radioactivity distribution in the cerebellum and temporal regions were well recovered when excluding the outside-FOV correction (figure 5, 6(c)‒(f)). The integrated transverse images obtained with the 15O-oxygen and 15O-carbon dioxide inhalation demonstrated reasonable quality and the border between cerebral spinal fluid and the white matter region were clearly visible. Identification of the carotid arterial lines as well as the separation of the carotid sinus from the temporal lobe can also be clearly seen, which has been difficult when using the previous PET scanners in 2D mode. Application of PSF and TOF reconstruction may contribute to the identification of the carotid-arterial and help in noninvasively extracting the arterial input function from the time-activity curve in this area, as demonstrated by an earlier work (Iguchi et al 2013). Further studies are needed in order to evaluate the adequacy of such an approach.
Ibaraki et al (Ibaraki et al 2011) demonstrated that the neck shield contributed to suppressing the random event rates, thus improving the NECR. They employed the energy-window-based scatter-correction technique, while the PET scanner in this experiment employed the scatter correction method of SSS. Although the shield did contribute to improved image noise in Ibaraki's experiment (Ibaraki et al 2011), other factors contributed in the experiment described here. The Siemens Biograph uses a 4.1 ns time window, contributing to a reduction in randoms due to the smaller window. Also, randoms smoothing is employed (Panin, 2008). Finally, the 435 keV lower-level threshold successfully rejects most of the scattered events from the activity in the lungs. Further study may be needed in order to identify the real outcome of the neck shield for the 15O-oxygen inhalation PET studies.
In this study, we evaluated the effects of eliminating the outside-FOV scatter compensation procedures of Watson et al (Watson et al 2004) on reconstructed images. Alternative procedures could be (a) to select the small threshold value optimal to identifying the wall of the inhalation system or (b) to employ a thicker material for the face mask to increase the attenuation factor. The former requires careful assessment of effective attenuation factors in all inhalation devices including the face mask and the latter also requires a careful evaluation in terms of minimizing the effects of possible movement of the absorption materials, as this might cause artifacts in the reconstructed images if the materials moved between the x-ray CT scan and the emission scan. These two approaches may, however, be realistic as a practical solution.
We have previously proposed a technique of intravenous injection of 15O2-hemoglobin as an alternative approach to the 15O-oxygen inhalation. This technique was successful in terms of reducing the random rates attributed to inhaling 15O2 in small animal studies (Magata et al 2003, Temma et al 2008, Temma et al 2010). However, intravenous injection requires critical procedures in order to guarantee safety in clinical settings. Inhalation is much easier in clinical studies. However, this alternative technique is still feasible if the degradation of the image quality and accuracy is still present. However, this study demonstrated that the quality is acceptable by using state-of-the-art PET devices. There could be an area where the injectable 15O2 approach might be beneficial over the inhalation in some of the PET examinations, such as in the thorax region, as discussed earlier (Temma et al 2010).
5. Conclusion
The present study demonstrated the accuracy of state-of-the-art 3D PET scanner for quantitative assessment of regional radioactivity concentration in the brain during 15O-oxygen inhalation, in both the phantom and normal volunteer experiments. It was shown that contribution to the scatter from outside-FOV is small in the brain PET study, and even a large amount of radioactivity in the lung region does not cause systematic errors. Scatter can be sufficiently compensated by means of SSS technique, provided that the entire radioactivity distribution in the brain, trachea and external inhalation system are included inside FOV. Large FOV acquisition in the axial direction is essential in order to maximize the accuracy of SSS scatter correction; otherwise, placing a facemask that contains strong radioactivity outside FOV results in large errors in brain regions.
Acknowledgement
The present work was supported by a grant from the Ministry of Health, Labor and Welfare, Japan. We are also indebted to staff from Department of Radiology, National Cerebral and Cardiovascular Center, Osaka, Japan.