Abstract
This study evaluated the effects of microcurrent application and 670 nm InGaP laser irradiation on wound healing in healthy and alloxan diabetic rats. The animals were divided into eight groups: healthy control (HC); diabetic control (DC); healthy treated with microcurrent (HMC); diabetic treated with microcurrent (DMC); healthy irradiated with laser (HL); diabetic irradiated with laser (DL); healthy receiving laser and microcurrent application (HLMC) and diabetic receiving laser and microcurrent application (DLMC). Wound samples were collected on days 2, 6, 10 and 14 of treatment for structural analysis, morphometry, and Western blotting to quantify the expression of TGF-β1 and VEGF. Comparison of animals receiving laser and microcurrent therapy showed a reduction in the number of inflammatory cells in diabetic animals, as well as an increase of fibroblasts in healthy animals and of newly formed vessels in healthy and diabetic animals. Expression of TGF-β1 was increased on day 6 in all groups, especially diabetic animals. A reduction in the expression of this protein was observed on day 10 in all groups. VEGF expression was higher on day 6 in treated and control diabetic animals when compared to healthy animals. Analysis of VEGF expression in the laser- and microcurrent-treated groups on day 10 showed a decrease in diabetic animals and an increase in healthy animals. In conclusion, laser therapy and microcurrent stimulation exert beneficial effects on wound healing in both healthy and diabetic animals.
Export citation and abstract BibTeX RIS
1. Introduction
Acute wounds normally heal in a very orderly and efficient manner characterized by four distinct, but overlapping phases: inflammation, proliferation and remodeling [1, 2]. The normal healing response begins the moment the tissue is injured. During the inflammatory phase the blood components spill into the site of injury, the platelets come into contact with exposed collagen and other elements of the extracellular matrix. In the hemostasis, the neutrophils then enter the wound site and begin the phagocytosis to remove bacteria and damaged tissue. It is also during this phase, the macrophages continue the process of phagocytosis and the fibroblasts migrate in to begin the proliferative phase and deposit new extracellular matrix. The new collagen matrix then becomes cross-linked and organized during the final remodeling phase. In order for this efficient and highly controlled repair process to take place, there are numerous cell-signaling events that are required and all of these stages are coordinated by specific cytokines and growth factors [2, 3].
One cytokine that plays an important role for the initiation of the healing cascade is transforming growth factor beta (TGF-β). This cytokine is secreted mainly by fibroblasts and keratinocytes and has a broad spectrum of action on all types of cells involved in the different stages of wound healing [4]. Several chemotactic factors are released in injured tissue, particularly TGF-β1, which recruits leukocytes that accumulate at the site of injury and induce the inflammatory phase [5].
TGF-β is considered to be a master control signal that regulates a host of fibroblast functions. Moreover, it has an effect on extracellular matrix deposition: it increases transcription of the genes for collagen, proteoglycans and fibronectin and the production in the matrix proteins and decreases the secretion of proteases responsible for their breakdown [2]. Members of the TGF-β family act as chemoattractants for neutrophils, macrophages and fibroblasts during wound healing, inducing various types of cells to produce more TGF-β1 and thus increasing the concentration of this cytokine in the inflammatory focus [6]. The result of these redundant signals is a vigorous response of the matrix producing cells to ensure a rapid deposition of new connective tissue at the injury site during the proliferative phase that follows the inflammatory phase [2].
A cytokine that stands out in the remodeling phase is the growth factor (VEGF). Due to the high metabolic activity at the wound site, there is an increasing demand for oxygen and nutrients. Local factors in the wound microenvironment, such as low pH, reduced oxygen tension and increased lactate actually initiate the release of factors needed to bring in a new blood supply. This process is called angiogenesis or neovascularization and is stimulated by this cytokine [2]. Members of the vascular endothelial growth factor (VEGF) family are signal proteins involved in the formation of new blood vessels. VEGF is one of the most potent angiogenic factors and is a key regulator of the physiological and pathological processes of blood vessel remodeling [7, 8]. The insufficient vascularization seen in diabetic patients is related to disturbances of angiogenesis and of VEGF expression. The latter are considered to be powerful proangiogenic factors [9].
Diseases such as diabetes mellitus (DM) are characterized by alterations in one or more phases of tissue repair. Disturbances in collagen synthesis and reepithelization and reduced angiogenesis are observed during the proliferation phase [10]. Chronic skin wounds are one of the most significant consequences of DM [11]. Several studies have investigated the mechanisms that regulate the wound repair process in patients with this disease [12, 13]. A decrease in the production of growth factors, excess proteolytic activity, and an increased microbial burden are possible factors that impair wound healing in DM [14, 15]. Furthermore, DM has been shown to interfere with fibroblast proliferation and collagen synthesis, exerting complex effects on the cellular mechanisms involved in wound healing [16]. In this respect, animals with diabetes experimentally induced by compounds such as alloxan monohydrate (2,4,5,6-tetraoxypyrimidine; 5,6-dioxyuracyl) are used as experimental models to elucidate the molecular and cellular mechanisms underlying this condition [17].
Various agents have been used as coadjuvantes to facilitate tissue repair, including low-level laser therapy (LLLT) [18, 19], ultrasound [20], administration of phytotherapeutic agents [21] microcurrent stimulation [22, 23] and cold plasma treatment [24–28]. There is growing interest in the application of cold atmospheric pressure plasma treatments in wound healing. This generates a low-power atmospheric discharge by the radio-frequency excitation of a mixture of helium and air. The gas remains at room temperature and treatment with this device is non-contact and entirely painless [24]. Potential advantages in wound healing have been demonstrated in vitro: the plasma does not necrotize the cells and does not affect the extracellular matrix, has clear bactericidal or bacteriostatic effects and stimulates fibroblast cells towards faster attachment and proliferation [25, 26]. Isbary et al [27] reported that the treatment with cold atmospheric plasma is an innovative promising tool to deal with chronic wounds because it reduces the bacterial load.
Electrical stimuli of different amplitudes and frequencies also are effective in promoting cell and tissue responses to experimentally induced injuries [28–30]. Microcurrent stimulation has been shown to trigger the onset and maintenance of numerous chemical and electrical reactions that occur during wound healing [23, 31]. Stimulation of live cells with low-intensity electrical currents directly affects the membrane potential and is associated with changes in ion gradients across the cell membrane, causing an increase in the synthesis of ATP followed by increased protein synthesis [32, 33].
The therapeutic effects of laser irradiation on different biological tissues have been demonstrated in vitro and in vivo and include an increase in local microcirculation, epithelial cell and fibroblast proliferation and collagen synthesis [34] and a reduction in the number of inflammatory cells [35], as well as analgesic effects [36]. In addition, LLLT has been shown to promote an increase of collagen fibers in wounds experimentally induced in diabetic and non-diabetic rats [37]. The results vary according to treatment mode, energy density, dose, wavelength, number and frequency of laser irradiation, and duration of treatment [38].
The present study compared the effects of microcurrent stimulation and LLLT on experimental wound healing in healthy and diabetic Wistar rats.
2. Material and methods
2.1. Animals
A total of 144 male Wistar rats (Rattus norvegicus) with a mean weight of 350 g were housed in individual polycarbonate cages at constant temperature (23 ± 2 °C) and humidity (55%) under a 12/12 h light/dark cycle, with free access to rations and water. The animals were obtained from the 'Professor Dr Luiz Edmundo de Magalhães' Experimental Animal Center, Centro Universitário Hermínio Ometto, UNIARARAS. All surgical and experimental procedures were approved by the Ethics Committee of UNIARARAS (Permit No. 087/2010) and were conducted in accordance with experimental guidelines and biodiversity rights [39, 40].
2.2. Experimental induction of diabetes
After a 24 h fast, the animals were injected intraperitoneally with a single dose of 150 mg kg−1 body weight alloxan (2,4,5,6-tetraoxohexahydropyrimidine) (Sigma Chemical Co., St Louis, MO, USA) diluted in 0.9% saline [41]. Next, the animals received glucose solution (10%) orally for 24 h to prevent complications of alloxan hypoglycemia [42]. Glucose concentration was determined with reagent strips using a portable blood glucose monitor (Accu-Chek Active®, AM Roche Diagnostics, USA) for 30 days to characterize the animal model. After this period, excision wounds were created in 72 animals with alloxan diabetes defined as glycemia >250 mg dl−1. The mean glycemia of these animals was 250 mg dl−1.
2.3. Experimental model
For wound production, a trichotomy was performed on the back of all animals 48 h before the surgical procedure. The animals were anesthetized with a combination of xylazine hydrochloride (0.2 mg kg−1) and ketamine hydrochloride (1 ml kg−1). Next, a skin fragment measuring 10 mm in diameter was excised through the epidermis and dermis down to the muscle fascia using a metal punch. For structural and morphometric analysis, the animals were divided into eight groups of 18 animals each: untreated healthy control animals (HC); untreated diabetic control animals (DC); healthy animals receiving microcurrent stimulation (HMC); diabetic animals receiving microcurrent stimulation (DMC); healthy animals receiving laser therapy (HL); diabetic animals receiving laser therapy (DL); healthy animals receiving intercalated applications of laser irradiation and microcurrent stimulation (HLMC); diabetic animals receiving intercalated applications of laser irradiation and microcurrent (DLMC).
Treatments were initiated 24 h after experimental injury. The animals were treated daily at the same time for 14 days. For treatment, the animals were immobilized but not sedated. A transcutaneous electrical stimulator (Physiotonus Microcurrent, Bioset Indústria de Tecnologia Eletrônica Ltda., Rio Claro, São Paulo, Brazil) was used for micro-galvanic stimulation (10 μA continuous current) by placing two metal electrodes with spherical tips (10 mm) in contact with the tissue around the excision site for 3 min (10 μA/3 min). A Physiolux Dual laser (Bioset) was used for laser therapy. This InGaP (indium gallide phosphide) diode laser emits a wavelength of 670 nm (visible red) with an output power of 30 mW, energy density of 4 J cm−2 and total energy dose of 0.36 J, with the beam covering an area of 0.073 cm2. The laser was applied for 12 s in the continuous mode. Irradiation was performed punctually (four points of 1 J cm−2, corresponding to 4 J cm−2 per day of treatment) using non-contact energy delivery. The laser was positioned at a distance of ±2 mm and at an angle of 90° in relation to the surface of the wound area. The apparatus was calibrated by the manufacturer (Bioset).
2.4. Collection and preparation of tissue samples for structural analysis, morphometry and Western blotting
On days 2, 6, 10 and 14 after experimental injury, three animals per group were killed by an overdose of the anesthetic and cervical dislocation and tissue samples were collected. For this purpose, a 10 mm area was delimited in the center of the wound to obtain standardized samples. The samples were fixed for structural analysis and morphometry. For analysis of protein expression by Western blotting, wound samples were collected from three animals per group only on days 6 and 10 of the experiment.
2.5. Structural analysis
The tissue fragments were fixed in 10% formaldehyde in Millonig buffer, pH 7.4, for 24 h at room temperature. After this period, the specimens were washed in buffer and submitted to standard processing for embedding in Paraplast™ (Histosec®, Merck). The blocks were cut into 6 μm longitudinal sections and stained with picrosirius-hematoxylin for the observation of collagen fiber organization by polarized light microscopy, with toluidine blue in McIlvaine buffer, pH 4.0, for analysis of epidermis, fibroblasts, inflammatory cells, and blood vessels, and with Dominici stain for detection of granulocytes. The images of the wound sections were captured and digitized with a Leica DM 2000 photomicroscope at the Laboratory of Micromorphology, Hermínio Ometto University Center, UNIARARAS.
2.6. Morphometry
Samples measuring 104 μm2 were removed from the central region of the wounds of three animals per group on days 2, 6, 10 and 14 and analyzed with the virtual Leica Image Measure™ grid for determination of the following morphometric parameters: tissue repair area (μm2), total number of cells (fibroblasts and inflammatory cells (n/104 μm2)), number of newly formed blood vessels (n/104 μm2), percentage of birefringent collagen fibers in the repair area (5), and epithelial thickness at the site of injury (μm). The Sigma Scan Pro 6.0™ program was used to measure the deposition of granulation tissue in the repair area. Birefringence in relation to the total area was measured by the picrosirius-polarization method for evaluation of the organization and maturation of collagen fibers [43]. The results were entered into spreadsheets (Biostat for Windows XP), and analyzed by ANOVA and the Tukey post-test for intragroup comparison and by the two-sample t-test for intergroup comparison (healthy and diabetic animals). A level of significance of 5% was adopted [44].
2.7. Western blotting
For protein extraction, the wound samples were chopped and homogenized with a Polytron homogenizer (PTA 20S model PT 10/35; Brinkmann Instruments, Westbury, NY, USA) in buffer containing 10 mM EDTA, 100 mM Trizma base, 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 100 mM sodium orthovanadate, 2 mM PMSF, 0.1 mg ml−1 aprotinin (Sigma) and deionized water, at maximum speed for 40 s. The extract was then centrifuged at 12 000 rpm for 20 min at 4 °C for removal of insoluble material. The supernatant was collected for determination of protein concentration by the biuret method (Protal colorimetric method, Laborlab, São Paulo, Brazil). Aliquots of the supernatant were treated with Laemmli buffer containing 100 mM DTT (Sigma). Samples containing 250 μg protein were boiled for 5 min and loaded onto 10% (VEGF, 40 kDa) and 12% (TGF-β1, 25 kDa) SDS-PAGE. The molecular mass standard used was Spectra Multicolor Broad Range Protein Ladder (Fermentas #1841). The gels were run in a Mini-Protean® apparatus (BioRad, CA, USA), and then transferred to nitrocellulose membranes (Hybond ECL, 0.45 μm). The membranes were washed in basal solution (1 M Trizma base, 5 M NaCl, 0.005% Tween 20, and deionized water) and incubated in blocking solution (basal solution plus 5% Molico® skim milk) to reduce nonspecific protein binding. After washing with basal solution, the membranes were incubated overnight at 4 °C with the anti-TGF-β1 (TB21, Santa Cruz Biotechnology, USA) and anti-VEGF (VG-1, Santa Cruz Biotechnology) antibodies, diluted 1:200. Next, the membranes were incubated with the secondary goat anti-mouse IgG1:HRP antibody diluted 1:1000 (Santa Cruz Biotechnology) for 2 h at room temperature. The reaction was developed using a chemiluminescent kit (SuperSignal® West Pico Chemiluminescent Substrate 34 080, Thermoscientific, USA). The membranes were exposed onto x-ray films (Kodak Medical x-ray, 18 cm × 24 cm). The band intensity was evaluated by densitometry using the Scion Image 4.0.3.2 software (Scion Co., USA). The densitometric values of VEGF and TGF signals were expressed relative to those proteins stained with Ponceau S, which were taken as 100%. The results were analyzed by ANOVA and Dunnett's post-test (p < 0.05) using the GraphPad Prism® 3.0 software.
3. Results
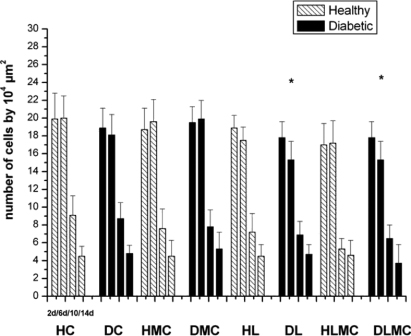
The tissue repair process was studied in healthy and diabetic animals by comparing the quantitative and qualitative results (inflammation, proliferation and tissue remodeling phase) obtained on days 2, 6, 10 and 14 after experimental injury. In healthy control (HC) animals, structural analysis revealed the presence of an inflammatory infiltrate in the repair area between day 2 and day 6 after experimental injury. However, few inflammatory cells were detected on day 10. The same features were observed in diabetic animals (DC) not submitted to any treatment (figures 1 and 2). In contrast, morphometric analysis comparing all groups revealed some differences in the total number of inflammatory cells. Significantly smaller numbers of these cells were observed in diabetic animals submitted to daily laser irradiation (DL) and in diabetic animals receiving alternating daily applications of microcurrent and laser (DLMC) when compared to the DC and DMC groups. Intragroup comparison of healthy animals showed no significant differences. In addition, no significant differences were observed between the groups of treated and untreated healthy and diabetic animals (figure 3).
Figure 1. Cross-sections of the excision wound area created on the back of healthy rats. HC: Untreated control; HMC: microcurrent stimulation (10 μA/3 min day−1); HL: laser therapy (InGaP diode laser, 670 nm, 4 J cm−2; 12 s d−1); HLMC: daily alternating applications of microcurrent and laser. For each group, samples were collected 2, 6, 10 and 14 days after experimental injury. The sections were stained with toluidine blue and examined under bright-field illumination. (*) Epidermis; (→) blood vessels; (▸) fibroblasts; ( ) inflammatory cells. Bar = 100 μm.
Download figure:
Standard imageFigure 2. Cross-sections of the excision wound area created on the back of diabetic rats. DC: Untreated control; DMC: microcurrent stimulation (10 μA/3 min d−1); DL: laser therapy (InGaP diode laser, 670 nm, 4 J cm−2; 12 s d−1); DLMC: daily alternating applications of microcurrent and laser. For each group, samples were collected 2, 6, 10 and 14 days after experimental injury. The sections were stained with toluidine blue and examined under bright-field illumination. (*) Epidermis; (→) blood vessels; (▸) fibroblasts; ( ) inflammatory cells. Bar = 100 μm.
Download figure:
Standard imageFigure 3. Total number of inflammatory cells (n/104 μm2) in the wound area of healthy (H) and diabetic (D) rats. HC and DC: untreated controls; HL and DL: laser therapy (InGaP diode laser, 670 nm, 4 J cm−2/day); HMC and DMC: microcurrent stimulation (10 μA/3 min d−1); HLMC and DLMC: combined microcurrent and laser treatment. Inflammatory cells were counted in samples collected on days 2 (2d), 6 (6d), 10 (10d), and 14 (14d) of the experiment. The results are the mean and standard deviation of each group and were compared by ANOVA with Tukey's post-test (p < 0.05) and by the two-sample t-test. * indicates significant intragroup differences (ANOVA) and Δ indicates significant intergroup differences (t-test).
Download figure:
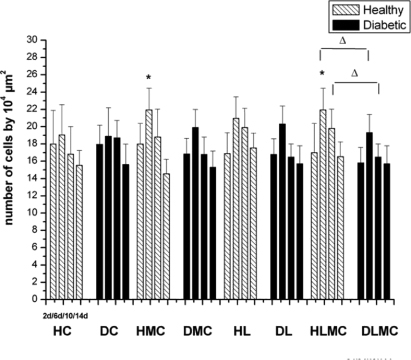
Standard imageStructural analysis showed the presence of fibroblasts between days 2 and 10 after experimental injury in the HC and DC groups (figures 1 and 2). Significantly larger numbers of fibroblasts were observed in samples collected on day 6 from animals of the HMC and HLMC groups when compared to group C. Although a larger number of fibroblasts were observed in diabetic animals receiving microcurrent stimulation (DMC), laser therapy (DL) and alternating daily applications of microcurrent and laser (DLMC), the differences were not significant when compared to the DC group. Comparison of healthy and diabetic animals showed significant differences in the reduction of the number of fibroblasts on days 6 and 10 only in the DLMC group when compared to the HLMC group (figure 4).
Figure 4. Total number of fibroblasts (n/104 μm2) in the wound area of healthy (H) and diabetic (D) rats. HC and DC: untreated controls; HL and DL: laser therapy (InGaP diode laser, 670 nm, 4 J cm−2/day); HMC and DMC: microcurrent stimulation (10 μA/3 min d−1); HLMC and DLMC: combined microcurrent and laser treatment. Fibroblasts were counted in samples collected on days 2 (2d), 6 (6d), 10 (10d), and 14 (14d) of the experiment. The results are the mean and standard deviation of each group and were compared by ANOVA with Tukey's post-test (p < 0.05) and by the two-sample t-test. * indicates significant intragroup differences (ANOVA) and Δ indicates significant intergroup differences (t-test).
Download figure:
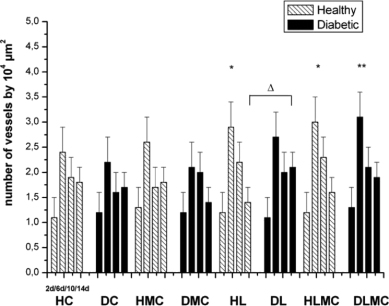
Standard imageIn group C, blood vessels were detected by day 2 after surgery, with the observation of arterioles and venules in the mid-portion of the repair area. The number of these vessels gradually increased from day 2 to day 6. The diameter of blood vessels observed in group DC between days 6 and 10 was apparently greater than the vessel diameter seen in group C (figures 1 and 2). The number of newly formed vessels on day 6 was significantly higher in the groups of healthy animals receiving daily laser therapy (HL) and alternating applications of microcurrent and laser (HLMC). Intragroup comparison of diabetic animals showed a larger number of arterioles and venules only in the DLMC group. When comparing healthy and diabetic animals, a significantly larger number of blood vessels were only observed in group DL compared to group HL (figure 5).
Figure 5. Total number of newly formed blood vessels (n/104 μm2) in the wound area of healthy (H) and diabetic (D) rats. HC and DC: untreated controls; HL and DL: laser therapy (InGaP diode laser, 670 nm, 4 J cm−2/day); HMC and DMC: microcurrent stimulation (10 μA/3 min d−1); HLMC and DLMC: combined microcurrent and laser treatment. Blood vessels were counted in samples collected on days 2 (2d), 6 (6d), 10 (10d), and 14 (14d) of the experiment. The results are the mean and standard deviation of each group and were compared by ANOVA with Tukey's post-test (p < 0.05) and by the two-sample t-test. * indicates significant intragroup differences (ANOVA) and Δ indicates significant intergroup differences (t-test).
Download figure:
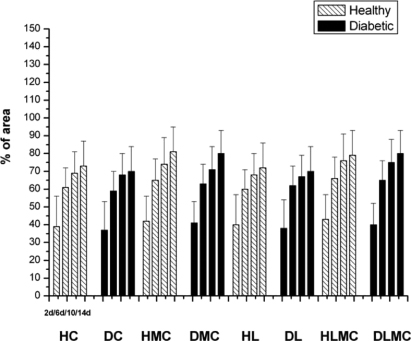
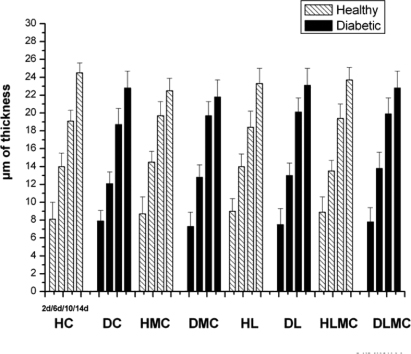
Standard imageThe structural organization of collagen fibers seen in samples of group C varied over the study period. On day 2 after injury, these fibers were thin and were found mainly close to blood vessels and the wound surface. Between days 6 and 10, these fibers were distributed throughout the dermis and were apparently thicker. Thick bundles of collagen fibers organized in a network at the wound edges were observed on day 14. Similar findings were obtained for samples of group DC (figure 6). Intragroup comparison of healthy and diabetic animals showed no significant differences in the area occupied by birefringent collagen fibers at any of the time points studied or between treatments. However, a progressive increase in the percentage of birefringent fibers was seen between day 2 and day 14 after experimental injury in all groups. Intergroup comparison revealed no significant differences (figure 7). With respect to epidermal thickness, intra- and intergroup comparison showed no significant differences at any of the time points studied (figure 8).
Figure 6. Cross-sections of the excision wound area created on the back of healthy (H) and diabetic (D) animals. HC and DC: untreated controls; HMC and DMC: microcurrent stimulation (10 μA/3 min d−1); HL and DL: laser therapy (InGaP diode laser, 670 nm, 4 J cm−2 per day); HLMC and DLMC: combined microcurrent and laser treatment. Samples were collected from each group on days 2 (2d), 6 (6d), 10 (10d), and 14 (14d) after experimental injury. The sections were stained with picrosirius-hematoxylin and analyzed under polarized light. (→) Birefringent collagen fibers. Bar = 100 μm.
Download figure:
Standard imageFigure 7. Percentage area occupied by mature collagen fibers in relation to the total repair area in skin wounds created in healthy (H) and diabetic (D) rats. HC and DC: untreated controls; HL and DL: laser therapy (InGaP diode laser, 670 nm, 4 J cm−2 per day); HMC and DMC: microcurrent stimulation (10 μA/3 min d−1); HLMC and DLMC: combined microcurrent and laser treatment. Collagen fibers were analyzed in samples collected on days 2 (2d), 6 (6d), 10 (10d), and 14 (14d) of the experiment. The results are the mean and standard deviation of each group and were compared by ANOVA with Tukey's post-test (p < 0.05) and by the two-sample t-test. * indicates significant intragroup differences (ANOVA) and Δ indicates significant intergroup differences (t-test).
Download figure:
Standard imageFigure 8. Epithelial thickness (μm) in the area of wounds created in healthy (H) and diabetic (D) rats. HC and DC: untreated controls; HL and DL: laser therapy (InGaP diode laser, 670 nm, 4 J cm−2 per day); HMC and DMC: microcurrent stimulation (10 μA/3 min d−1); HLMC and DLMC: combined microcurrent and laser treatment. Epithelial thickness was measured in samples collected on days 2 (2d), 6 (6d), 10 (10d), and 14 (14d) of the experiment. The results are the mean and standard deviation of each group and were compared by ANOVA with Tukey's post-test (p < 0.05) and by the two-sample t-test. * indicates significant intragroup differences (ANOVA) and Δ indicates significant intergroup differences (t-test).
Download figure:
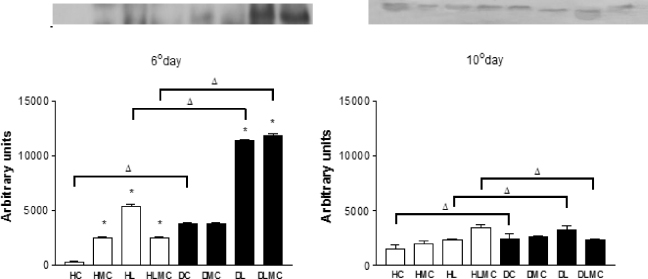
Standard imageAnalysis of the expression of TGF-β1 and VEGF by Western blotting and densitometry revealed some differences (figures 9 and 10). Variations in the levels of TGF-β1 expression between the different treatments were observed in healthy and diabetic animals on day 6 (figure 9). All treatments exerted significant effects on the expression of this protein in healthy treated animals when compared to the untreated controls. In diabetic animals, expression of TGF-β1 was significantly higher on day 6 in the DL and DLMC groups when compared to control (DC group). However when comparing intragroups, the data obtained after day 10 indicated that the expression of TGF-β1 in samples of both healthy and diabetic animals showed a decrease in the expression of this protein in the different treatments in relation to day 6, especially for groups DL and DLMC of diabetic animals (table I).
Figure 9. Quantification of the expression of TGF-β1 in wounds of healthy (white columns) and diabetic (black columns) animals by densitometric analysis on x-ray films using the Scion Image software. Values are expressed as the mean ± standard deviation. * p < 0.05: significant difference compared to healthy and diabetic controls (column C and column DC) (Dunnett test); Δ p < 0.05: intergroup differences (t-test).
Download figure:
Standard imageFigure 10 shows the expression of VEGF on days 6 and 10 after experimental injury in healthy and diabetic animals receiving the different treatments. In healthy animals, significant expression of VEGF was observed on day 6 for all treatments when compared to control (group HC). In contrast, no difference in VEGF expression was observed for diabetic animals submitted to the different treatments when compared to the control group (DC). However, expression of VEGF was higher in group DC than in group HC. A significant increase of VEGF expression was observed on day 10 in healthy animals submitted to the different treatments when compared to control, whereas the expression of this protein was reduced in diabetic animals submitted to laser therapy and laser therapy combined with microcurrent stimulation (table II).
Figure 10. Quantification of the expression of VEGF in wounds of healthy (white columns) and diabetic (black columns) animals by densitometric analysis on x-ray films using the Scion Image software. Values are expressed as the mean ± standard deviation. * p < 0.05: significant difference compared to healthy and diabetic controls (column C and column DC) (Dunnett test); Δ p < 0.05: intergroup differences (t-test).
Download figure:
Standard image4. Discussion
Structural analysis showed no significant differences in the inflammatory phase of the healing process between healthy and diabetic animals. The impaired tissue repair seen in patients with DM is associated with alterations in the mechanisms underlying one or more phases of wound repair [45]. In general, prolongation of the inflammatory phase delays wound healing [46]. In the present study, daily laser therapy and alternating microcurrent and laser application led to a significant reduction in the number of inflammatory cells on day 6 after experimental injury in diabetic animals. LLLT seems to modulate inflammation in various tissues, effectively promoting a reduction of inflammatory infiltration and acceleration of the tissue repair process [35, 47, 48]. The biomodulatory effects of laser irradiation are probably associated with an increase in the production of growth factors [49] and a reduction of plasma proinflammatory cytokines [50] and prostaglandins [51]. Dall Agnol et al [52] treated wounds induced in diabetic and non-diabetic rats with LLLT (InGaAlP, 660 nm) and observed a significant reduction in the number of inflammatory cells in the two groups compared to control. Furthermore, several studies have shown the effects of low-amperage electrical currents on experimentally induced wounds [22, 23, 28–30, 53]. Kaur et al [54] demonstrated that a galvanic current reduces the inflammatory response in edemas induced in the ear of mice. Longo [55] also reported that laser therapy has anti-inflammatory effects, with stimulation of beta cells, of chromaffin cells, and of microcirculation. In the present study, analysis of the number of inflammatory cells in wound samples obtained from the experimental groups showed no significant differences between healthy and diabetic animals.
Intragroup comparison showed a significantly larger number of fibroblasts on day 6 after experimental injury in healthy animals receiving microcurrent stimulation and microcurrent + laser therapy when compared to the control group. Gentzkow [56] found that electrical stimulation promotes the proliferation of fibroblasts and has beneficial effects on the healing of dermal wounds and Nayak et al [57] observed an increase of collagen production as a consequence of the modulation of fibroblast proliferation in excision wounds of Wistar rats after LLLT.
In the present study, a significant increase in the number of fibroblasts was observed in wounds of healthy animals treated with laser and microcurrent compared to control. In addition, the number of these cells was significantly lower in the DLMC group on days 6 and 10 when compared to the HLMC group. The latter finding is probably related to the altered healing process seen in DM, which is characterized by impaired differentiation of fibroblasts into myofibroblasts, compromising wound contraction and remodeling [58]. Thus, laser and microcurrent treatment elicited different responses in terms of fibroblast proliferation in the wounds of diabetic and healthy animals.
With respect to new blood vessel formation, a significant increase was observed on day 6 of the experiment in the HL and HLMC groups, whereas among diabetic animals only those submitted to the combination treatment (DLMC) presented a significant response. Quantitative analysis also showed a larger number of blood vessels in the DL group when compared to the HL group on day 14. Several studies have demonstrated that LLLT increases local microcirculation [59] and angiogenesis [60]. Furthermore, application of low-level electrical currents increased the number of newly formed vessels during experimental wound healing in Wistar rats [29, 30]. Reduced angiogenesis during the proliferative phase of wound healing in diabetics [10], disturbances of extracellular matrix synthesis by fibroblasts and skin microvascularization promoting tissue ischemia, and chronic diabetic wounds have been reported [61, 62]. In the present study, alternating applications of microcurrent stimulation and laser irradiation favored vascularization in the DLMC group (day 6), which was significantly higher than that observed in the other diabetic groups and similar to that seen in the HLMC group.
Although the percentage of birefringent collagen fibers progressively increased throughout the healing process from day 2 to day 14 after experimental injury, intra- or intergroup comparison revealed no significant responses in any of the treatment groups at the different time points. These results agree with those reported by Neves et al [19], who studied the effects of different power output settings of LLLT on the repair of the rat calcaneal tendon and found no significant difference in the percentage of birefringent collagen fibers between the different treatments. In contrast, Carvalho et al [18] reported a significant increase in the percentage of collagen fibers in diabetic rats submitted to LLLT (InGAlP, 660 nm). Bastos et al [63] also reported that applications of an infrared laser at 830 nm were efficient when the aim is a good organization, aggregation, and alignment of the collagen bundles on tendon healing.
The different treatments led to an increase in the expression of TGF-β1 in healthy and diabetic animals, except for group DMC, particularly on day 6 after experimental injury. TGF-β1 is a chemoattractant for neutrophils and macrophages and plays an important role during the early stages of wound healing. Higher expression of this protein was observed in group DC compared to group C on day 6. This finding is probably related to the fact that tissue repair is slower and the inflammatory phase is prolonged in diabetic individuals [64]. Significantly higher expression of TGF-β1 was observed in the DL and DLMC group compared to the DC group, indicating that laser and microcurrent treatment stimulated the expression of this protein and the evolution of tissue repair.
Densitometry showed a decrease in the expression of TGF-β1 in treated and untreated healthy and diabetic animals on day 10 when compared to day 6. Differences in the expression of the three isoforms of the TGF-β family during tissue repair have been reported in the literature [65, 66]. O'Kane and Fergusson [67] demonstrated the expression of TGF-β1 and TGF-β2 during the early stages of wound healing and an increased expression of TGF-β3 during the final stages. These findings may explain the lower expression of TGF-β1 observed in all healthy and diabetic groups on day 10 after experimental injury.
Expression of VEGF was found to be higher in all diabetic groups on day 6 when compared to the respective groups of healthy animals. This finding might be related to the tissue hypoxia commonly seen in the skin of diabetic individuals as a consequence of impaired microcirculation [62]. Diabetic individuals present insufficient vascularization related to impaired angiogenesis. That consequent tissue hypoxia significantly increases the expression of VEGF may be responsible for improved tissue repair [68]. Galiano et al [69] demonstrated that treatment of db/db mice with VEGF favors new blood vessel formation at the site of injury through increased angiogenesis, suggesting that this protein can promote the healing of a wide variety of chronic wounds, particularly in diabetes and during aging. On day 10 after experimental injury, the expression of VEGF was increased in all groups of healthy animals compared to control and was decreased in diabetic animals, particularly groups DL and DLMC, when compared to group DC. The reduced expression of this protein in diabetic animals might be related to the smaller number of fibroblasts found in diabetic wounds, since these cells are producers of this potent angiogenic cytokine [8].
In all parameters studied, we observed that the application of alternating microcurrent and laser promoted positive responses in excisional injury repair. This suggests a beneficial effect of these two applications. The mechanisms of these kinds of therapy are different. The application of the microcurrent produces electrical signals like those produced by the physiological electromagnetic field, normalizing the natural bioelectricity altered by the lesion, increasing ATP production, protein synthesis, oxygenation and ion exchange [70], whereas the application of the laser promotes selective stimulation of mitochondria, increasing the cellular metabolism and aiding tissue repair [71]. The alternating application of these techniques proved to be more effective, probably due to their differential effects on the various mechanisms necessary for the development of tissue repair. In both are observed parameters such as wavelength, frequency, dose, potency, intensity, time interval and number of applications, which operate within a range that does not compromise the physiological microarchitectural tissue.
5. Summary
Comparison of animals receiving laser and microcurrent treatment showed a reduction in the number of inflammatory cells in diabetic animals, an increase in the number of fibroblasts in healthy animals, and an increase in the number of newly formed blood vessels in both healthy and diabetic animals. The expression of TGF-β1 was increased in samples collected on day 6 for all treatments, especially in diabetic animals, and was decreased on day 10 in all groups. Higher expression of VEGF was observed on day 6 in wounds of treated and control diabetic animals when compared to healthy animals. Analysis of VEGF expression in the laser- and microcurrent-treated groups on day 10 showed a decrease in diabetic animals and an increase in healthy animals. In conclusion, laser therapy and microcurrent stimulation exert beneficial effects on wound healing in both healthy and diabetic animals.