Abstract
Three-dimensional (3D) bioprinting combines biomaterials, cells and functional components into complex living tissues. Herein, we assembled function-control modules into cell-laden scaffolds using 3D bioprinting. A customized 3D printer was able to tune the microstructure of printed bone mesenchymal stem cell (BMSC)-laden methacrylamide gelatin scaffolds at the micrometer scale. For example, the pore size was adjusted to 282 ± 32 μm and 363 ± 60 μm. To match the requirements of the printing nozzle, collagen microfibers with a length of 22 ± 13 μm were prepared with a high-speed crusher. Collagen microfibers bound bone morphogenetic protein 2 (BMP2) with a collagen binding domain (CBD) as differentiation-control module, from which BMP2 was able to be controllably released. The differentiation behaviors of BMSCs in the printed scaffolds were compared in three microenvironments: samples without CBD-BMP2-collagen microfibers in the growth medium, samples without microfibers in the osteogenic medium and samples with microfibers in the growth medium. The results indicated that BMSCs showed high cell viability (>90%) during printing; CBD-BMP2-collagen microfibers induced BMSC differentiation into osteocytes within 14 days more efficiently than the osteogenic medium. Our studies suggest that these function-control modules are attractive biomaterials and have potential applications in 3D bioprinting.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Three-dimensional (3D) bioprinting is more complex than the non-biological printing [1], which involves many technical challenges of biomaterial fabrication [2], growth factor (GF) delivery, cell selection and so on [3]. Thus, it is crucial to integrate technologies from biomaterial science, cell biology, engineering and other fields. The key challenge is to reconstruct the niches composed of extracellular matrix (ECM) components and multiple cell types to recapitulate biological function [1, 4–10]. 3D printed ECM should provide adhesive, proliferative and migration-permissive microenvironments for cells to construct tissues or organs with structures and functions similar to natural ones [11].
Recently, ECM hydrogel analogs have been used to regulate cell behaviors of growth, differentiation and apoptosis [12–15]. The active groups in the polymer chains of hydrogels can be modified with ECM components to mimic specific cell niches [16]. On the other hand, as signal molecules, GFs are usually loaded into the hydrogel network for cell growth and tissue reconstruction. Therefore, controllable delivering GFs is currently considered as a main concern for developing functional hydrogel scaffolds to avoid some potential adverse effects [17–20]. However, GF delivery could not be well controlled via the reported vehicles of polymer micro-spheres [21] and water/oil emulsions [22]. In our previous studies, a 'green' and controllable method was developed to deliver GFs in the hydrogel network. TKKTLRT, a short collagen binding domain (CBD), derived from mammalian collagenase, was designed to make GFs specially bind collagen [23]. The recombinant proteins could be delivered with collagen in a site-specific manner [24–29].
In this study, we assembled collagen-binding domain-bone morphogenetic protein 2 (CBD-BMP2)-collagen microfibers as differentiation-control modules into bone mesenchymal stem cell (BMSC)-laden methacrylamide gelatin (MG) scaffolds via a customized 3D printer. Collagen microfibers were prepared with a high-speed crusher that was compatible with the printing nozzle. BMP2 was able to be controllably released through CBD-BMP2-binding collagen microfibers. BMSC differentiation in the printed scaffolds was compared with different culture microenvironments, confirming CBD-BMP2-collagen microfibers as differentiation-control modules.
2. Materials and methods
2.1. Materials
The collagen films were provided by Zhenghai Biotechnology, Inc. (Shandong, China). Gelatin from porcine skin was purchased from Sigma. Methacrylic anhydride was purchased from Aldrich. Standard BMP2 was obtained from Neobioscience Technology (Beijing, China). Other reagents without mentioned were purchased from Sinopharm Chemical Reagent Co., Ltd. The water was purified by a three-stage Millipore Milli-Q plus 185-purification system in all experiments.
2.2. Preparation of MG
MG was prepared as reported [30, 31]. Briefly, 0.6 mL of methacrylic anhydride was added to 10 mL of 10 wt.% gelatin solution and stirred for 4 h at 50 °C. The mixture was cooled to 40 °C to quench the reaction and added in a 1:4 ratio (V/V) to ice-cold acetone to form a precipitate. A 10 wt. % MG solution was placed in dialysis tubing with a molecular weight of 8–14 kDa, and dialyzed against deionized (DI) water for 3 days at 40 °C. The dialyzed MG was lyophilized for 4 days and stored at −20 °C until use.
2.3. Preparation of CBD-BMP2
CBD-BMP2 was prepared as described previously [25]. The CBD-BMP2 gene was firstly amplified by the polymerase chain reaction to insert into the pET-28a vector (Novagen, Madison). Protein expression was induced by adding 1 mM isopropyl-β-D-thiogalactopyranoside at 25 °C for 8 h. The recombinant CBD-BMP2 protein was purified via nickel chelate chromatography (Amersham Biosciences, Piscataway).
2.4. Preparation of collagen microfibers
To prepare collagen microfibers, collagen films were first cut into cm-sized pieces with a crushing-particle desk crusher (RZ210D, Anrui). These collagen pieces were further crushed into fibers at 22 000 r min−1 through an air-bleed crusher (FDV, Youqi Co., Ltd). To match the requirements of the printing nozzle, the collagen fibers were dispersed in water and selected through 100 mesh (150 μm), 200 mesh (75 μm) and 400 mesh (38 μm) nylon meshes (Beijing Kelong Biomedicine Technology Co., Ltd) to obtain different μm-sized microfibers. The lyophilized microfibers were sterilized with Co60 radiation before use. The collagen microfibers passed through 400 mesh (38 μm) nylon meshes were measured with Image J software and used in the following experiments.
2.5. Process of binding CBD-BMP2 into collagen microfibers
The CBD-BMP2-collagen microfibers used in 3D bioprinting were fabricated by dispersing the collagen microfibers into a CBD-BMP2 solution. Typically, 1 mg of collagen microfibers was dispersed into 0.5 mL of 100 μg mL−1 CBD-BMP2 solution for 30 min. To characterize the process of binding CBD-BMP2 onto collagen microfibers, the loaded CBD-BMP2 was labeled with an anti-human BMP monoclonal antibody (sc-6895, goat polyclonal IgG, Santa Cruz Biotechnology) and green fluorescein conjugated affinity-purified anti-goat IgG (Alexa Fluor® 488 donkey anti-goat IgG, Life technology). The bound collagen microfibers were observed under a Leica TCS SP5 confocal microscope (Leica Microsystems, Germany) with a green excitation light of 488 nm.
To test the controllable release of CBD-BMP2 from collagen microfibers, the BMP2 or CBD-BMP2 concentration was measured with a human BMP2 ELISA kit (EHC172, Neobioscience Technology). The standard curve of the BMP2 solution was obtained on a scale of 0–1 ng mL−1. The loaded sample was immersed into 200 μL of PBS solution for the release test. At predetermined time intervals of 1, 2, 3, 4 and 5 days, the BMP2 concentration in PBS solution was measured to calculate the amount of released BMP2. Then, the sample was soaked into a fresh PBS solution for the next measurement. The BMP2 release ratio was calculated as the following formula: BMP2 release ratio (%) = Mt/M0 × 100%, where M0 is the mass of original loaded BMP2 in the specimen and Mt is the total mass of released BMP2 in PBS solution at time t.
2.6. Cell isolation, culturing and seeding
The animal surgeries were performed as described previously [32] in accordance with NIH guidelines (NIH Publication No. 85-23 Rev. 1985). BMSCs were harvested from 4-week-old male adult Sprague-Dawley (SD) rats and cultured in the incubator (STERI 371, Thermo Electron Co.). The 3–5 passage BMSCs were used in the follow-up experiments. The cell viability was recorded via trypan blue staining in an automatic cell counter (IC1000, Countstar).
2.7. 3D Bioprinting
A customized 3D printer (Shenyang Institute of Automation, Chinese Academy of Sciences) with four independent, z-axis-controlled ink reservoirs was used in our study. The designed CAD/CAM models are translated into a numerical code (G code) by the bioprinting software of Slic3r V 1.1.6, CIMCO Edit 4.33.33 and PmacCtrl. exe. Several types of nozzles were used, such as stainless steel nozzles (e.g., the 27 G nozzle with an inner diameter of 210 μm). The bioprinter deposits cell-laden or CBD-BMP2-collagen microfiber-laden hydrogel precursors mechanically on a stationary platform. The two-stage syringe heating mantle was used to control a homogeneous plotting temperature. The stationary platform was equipped with a refrigerator to cool the platform below the MG gelling point.
To photo-crosslink the MG hydrogel, 0.5–5 wt. % VA-086 (Wuhan Fude Chemical co., Ltd) or 0.3–2 wt. % of photoinitiator I2959 (BASF) was optimized and added into the printing inks. All of the inks were filtered through a 0.22 μm filter and mixed with cells at 37 °C for printing. The printed structure was photo-crosslinked with a UV light source for 1–5 min (Stylus UV Alkaline Battery-Powered Pen Light).
To test CBD-BMP2-collagen microfibers as differentiation-control modules in printed BMSC-laden scaffolds, the printed scaffolds were cultured in three different conditions: samples without CBD-BMP2-collagen microfibers in the growth medium as control group 1, samples without microfibers in the osteogenic medium as control group 2 and samples with microfibers in the growth medium as the test group. The growth medium of L-DMEM contains 10% fetal bovine serum (FBS). The osteogenic medium consisted of L-DMEM, 10% FBS, 100 nmol L−1 dexamethasone, 10 mmol L−1 sodium b-glycerophosphate and 0.05 mmol L−1 L-ascorbic acid 2-phosphate. The osteogenic medium was changed every 2 days.
2.8. Scanning electron microscopy (SEM) analysis
The scaffolds were dehydrated with sequential increasing ethanol solution as reported [14]. Freeze-dried scaffolds, coated with gold, were observe on a HITACHI S-3000N scanning electron microscope (Hitachi Science Systems, Japan). The pore size was measured with Image J software.
2.9. Live/dead cell staining
A live/dead cell assay was conducted on days 0, 7, 14 and 28. Cell-laden scaffolds were washed in PBS and incubated for 30 min at 37 °C with 2 mM calcein-AM (Sigma), 2 mM propidium iodide (PI, Sigma) and 2 mM 4, 6-diamino-2-phenyl indole (DAPI, Sigma) in PBS. After incubation, the samples were rinsed and viewed using a Leica TCS SP5 confocal microscope (Leica Microsystems, Germany) with the blue, green and red excitation lights of 358 nm, 488 nm and 562 nm respectively. Cell viability was calculated via the live/dead cell staining images, where live cells, dead cells and nuclei displayed green, red and blue respectively.
2.10. Total DNA quantification for cell proliferation
The samples, cultured for 7 or 28 days, were used for DNA quantification as reported [14]. The total DNA in the digested sample was extracted by a DNeasy Blood and Tissue kit (69504, QIAGEN, Germany) and was quantified on a biophotometer (Gene Quant Pro).
2.11. RNA isolation and quantitative real-time PCR
Total RNA was extracted using TRIzol (Invitrogen) reagents as the manufacture's protocol [32]. Quantitative PCR (qPCR) was performed through Power SYBR® Green PCR Master Mix (Applied Biosystems, UK) on a CFX96 system (Bio-Rad, USA). The target genes and primer sequences are listed in table 1.
Table 1. Primers designed for RT-PCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Alkaline phosphatase (ALP) | CCGATCGGGACTGGTACTC | TCAGTTCTGTTCTTGGGGTACA |
| Bone sialoprotein (BSP) | CGGCCACGCTACTTTCTTTA | CCCTCCTCCTCCGAACTATC |
| Osteocalcin (OCN) | GAGGGCAGTAAGGTGGTGAA | GTCCGCTAGCTCGTCACAAT |
| Collagen type I (COLLA1) | CATGTTCAGCTTTGTGGACCT | GGTTTCCACGTCTCACCATT |
| GAPDH | AGAGACAGCCGCATCTTCTTG | ACCGACCTTCACCATCTTGTCTA |
2.12. Alkaline phosphatase (ALP) staining and activity assay
After culturing for 14 days, the scaffolds were rinsed three times with PBS for ALP staining via a BCIP/NBT ALP color development kit (Beyotime, China). PBS containing 0.1% TritonX-100 was used to release cytoplasmic ALP. The total protein concentration was determined via a BCA Protein Assay Kit (Pierce, USA). ALP activity was quantified via an ALP assay kit (Beyotime, China) and observed under a light microscope (DXM1200C Digital Camera, Nikon).
2.13. Alizarin red S staining and determination of calcium content
After washing twice with PBS, the samples were immersed in 95 v/v % ethanol for 15 min and stained with 1 wt./v % Alizarin red S (Sigma) for 20 min to observe under a light microscope (DXM1200C Digital Camera, Nikon). The calcium content was detected via a QuantiChrom Calcium Assay Kit (DICA-048, BioAssay Systems).
2.14. Statistical analysis
All of the experiments were performed at least three times and the values were expressed as the mean  standard deviation (SD). Statistical analysis was performed with Student's t-test software. Differences were considered significant when *p < 0.05 or **p < 0.01.
standard deviation (SD). Statistical analysis was performed with Student's t-test software. Differences were considered significant when *p < 0.05 or **p < 0.01.
3. Results and discussion
3.1. Customized 3D printer
3D bioprinting supplies a flexible automated on-demand platform to fabricate complex living architectures [1]. During printing, biomaterials with living cells, GFs and even drugs can be precisely oriented for tissue engineering, regenerative medicine and so on. Several techniques are applied in 3D biopriting, such as valve-based printing and inkjet-based printing. Among them, microextrusion has been widely used in 3D bioprinter development [33–35]. The microextrusion bioprinters control the extrusion of a material robotically with a microextrusion nozzle. The stage or microextrusion head moves along x- and y-axis, in this way a layer is deposited as a foundation for the next layer when the extrusion head rising along z-axis.
Our customized microextrusion bioprinter is shown in figure 1, consisting of a material-handling and dispensing stage equipped with a temperature-controlled system. In four independently controlled cell-dispensing channels, BMSCs, MG and CBD-BMP2-collagen microfibers can be placed for printing (figure 1(A)). Electromechanical valves operate each dispenser, which is mounted on a three-axis robotic stage for the micrometer resolution (figure 1(B)). The movement of printing nozzle is displayed in Video 1. In the printing process (figure 1(C)), the solution was dispensed and layer-by-layer printed as the CAD/CAM software. Later, the MG hydrogel was photo-crosslinked via a UV light source. Some key parameters, including initial MG concentration (5–20 wt./v%), cell number (1–5 × 106 cells/mL), CBD-BMP2-collagen microfiber content (0.01–0.1 wt. %), applied pressure (0–5 bar), temperature (4–37 °C), plotting speed (0–5 mm s−1), plotting infill (0.7–1.2 mm), plotting height (0.01–0.09 mm) and syringe diameter (5.5–7.5 mm), were studied and optimized during 3D bioprinting.
Figure 1. Fabrication of 3D scaffolds using customized 3D printer. (A) 3D printer equipped with refrigeration. (B) Printing nozzle. (C) Schematic of the printing process and photo-crosslinking process.
Download figure:
Standard image High-resolution imageThe microextrusion bioprinting has two common dispensing systems: pneumatic [36] and mechanical (piston or screw) [37, 38]. Compared with pneumatic systems, mechanical dispensing systems could directly control over the material flow. Especially, screw-based systems are more suitable to dispense hydrogels with higher viscosities [39]. We improved the printer equipped with screw-based systems for printing at the micrometer scale to afford the flexibility of spatial control in the scaffold microstructure. The front and sectional images of the printed scaffolds (20 mm × 20 mm × 2 mm) are shown in figures 2(A) and (B), respectively. Observed under a light microscope, the grid structure was precisely printed as the designed pattern with a plotting infill parameter of 900 μm (figures 2(C)) and 1000 μm (figure 2(D)). SEM images further display the microstructure printed as different designed patterns (figures 2(E) and (H)), whereas the magnified illustrations show the layer-by-layer microstructure (figures 2(F) and (I)). The pore size distribution is even. Notably, the pore size can be adjusted to 282 ± 32 μm and 363 ± 60 μm (figures 2(G) and (J)). Thus, the microextrusion bioprinter is able to offer a promising method to mimick the complexity of the native microenvironment.
Figure 2. Observation of printed methacrylamide gelatin scaffolds. (A) Front image of printed scaffold, scale bar = 5 mm. (B) Sectional image of printed scaffold, scale bar = 5 mm. (C) Printed scaffold with pore sizes of 900 μm was observed under transmission light, scale bar = 100 μm. (D) Printed scaffold with pore sizes of 1000 μm was observed under transmission light, scale bar = 100 μm. (E) SEM image of printed scaffolds with a pore size of 363 ± 60 μm, scale bar = 200 μm. (F) SEM image of a magnified pore in (E), scale bar = 50 μm. (G) The pore size distribution of printed scaffolds in (E) was shown. (H) SEM image of printed scaffolds with a pore size of 282 ± 32 μm, scale bar = 200 μm. (I) SEM image of a magnified pore in (H), scale bar = 50 μm. (J) The pore size distribution of printed scaffolds in (H) was shown.
Download figure:
Standard image High-resolution image3.2. CBD-BMP2-collagen microfibers
The differentiating capability of stem cells could be limited due to the lack of natural GFs. For example, bone morphogenetic proteins (BMPs), platelet-derived growth factor and transformation growth factors (TGF) have shown some supporting effect in both osteoinductivity and osteogenicity [40, 41]. Among them, BMPs can regulate the functionality and differentiation of cells during bone formation and healing [42]. BMP2, belonged to BMP family, has received three US Food and Drug Administration approvals and been used to induce the osteogenic differentiation of osteoblasts and multipotential mesenchymal cells [43]. Thus, the BMP2 protein has been widely applied in various bone-related treatments [44]. However, direct injection of soluble BMPs to the affected site is not effective enough for the bone regeneration due to its rapid diffusion. Hence, function-control modules have been assembled into the scaffolds in 3D bioprinting, in order to provide a sustained delivery of GFs in hydrogel network [45].
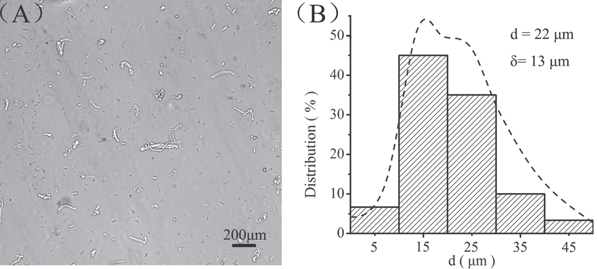
An absorbable collagen sponge with recombinant human BMP2 has been used clinically and was shown to improve bone healing at a therapeutic dose of 12 mg per treatment [46, 47]. However, no specific binding affinity was found between the collagen sponge and BMP2, which caused a 30% burst release upon initial implantation [48]. To improve the efficacy of BMP2-based therapies and limit adverse responses, new protein products should prolong protein retention and reduce the therapeutic dose of GFs. Therefore, we developed CBD-BMP2-collagen microfibers. Collagen is important for maintaining the biological and structural integrity of the ECM. During these processes, collagen functions as a highly organized, dynamic remodeled 3D architecture, which provides anchorage sites and structural guidance for cell adhesion, migration and differentiation [49]. Collagen microfibers compatible with the printing nozzle were prepared by the high-speed crusher and selected through 400 mesh (38 μm) nylon meshes. Even collagen microfibers with a length of 22 ± 13 μm are shown in figure 3(A), while figure 3(B) shows their length distribution. Because 27 G stainless steel nozzles have an inner diameter of 210 μm in our 3D printer, the collagen microfibers was able to be printed through the nozzle.
Figure 3. Fabrication of collagen microfibers. (A) Collagen microfibers with a length of 22 ± 13 μm to match the printing nozzle requirements, scale bar = 200 μm. (B) The length distribution of collagen microfibers.
Download figure:
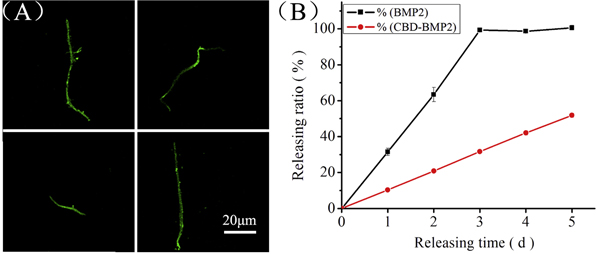
Standard image High-resolution imageTo fuse BMP2 onto the collagen microfibers, a short collagen-binding domain, derived from mammalian collagenase was designed [25]. To confirm CBD-BMP2 binding to collagen microfibers, the loaded CBD-BMP2 was labeled with an anti-human BMP monoclonal antibody and then with green fluorescein conjugated to affinity-purified anti-goat IgG. Under the confocal microscope, the labeled CBD-BMP2-collagen microfibers displayed green fluorescence (figure 4(A)). CBD-BMP2 releasing behavior from collagen microfibers was compared with that of BMP2 in figure 4(B). Most of the BMP2 (99.3 ± 0.7%) was released during the first three days, while the releasing ratio of CBD-BMP2 was 51.9 ± 1.1% at day 5. These results suggest that the controlled release of CBD-BMP2 could be achieved using collagen microfibers.
Figure 4. Characterization of CBD-BMP2-collagen microfibers. (A) CBD-BMP2-collagen microfibers labeled with green fluorescence observed under the confocal microscope, scale bar = 20 μm. (B) BMP2 and CBD-BMP2 release curve from collagen microfibers.
Download figure:
Standard image High-resolution image3.3. Printed hydrogel scaffolds
3D bioprinting technology should take up the challenges of maintaining the cell viability and long-term functionality. To reproduce the complex microenvironments of ECMs and target cells, biomaterial fabrication surface resolution and cell viability should be considered in 3D bioprinting [50]. The biological demands accordingly define a special standard to select suitable biomaterials used in the field of regenerative medicine [51]. The natural biomaterials, such as alginate and gelatin, often harbor bioactivity and have similar structures to human ECMs for 3D bioprinting applications. Meanwhile, bioprinting technology requires proper crosslinking mechanisms to achieve desired spatial and temporal control [12]. In short term, the printed scaffolds should retain the initial mechanical properties. For example, tissue structures, such as pores, channels and networks, should not collapse. Furthermore, the printed scaffolds should be biocompatible, focusing on cytocompatibility, for future transplantation.
In the present study, we synthesized MG as a bulk matrix and cell carrier [30, 31, 52]. Gelatin is denatured collagen and selected for its low-cost, simple processing and biocompatibility. Gelatin was modified with photopolymerizable methacrylate (MA) groups to obtain MG, which allowed the matrix to covalently cross-link via UV light after printing. We specifically produced a concentrated ECM-like ink by dissolving 10 wt./v % MG in the growth medium. The ink was a low viscosity fluid at ≥23 °C. The ink underwent gelation into a clear viscoelastic matrix material after cooling below 23 °C. The ink elasticity increases inversely with temperature in accordance with typical conditions for printing. Therefore, we used this aqueous MG system to create cell-laden inks for 3D bioprinting.
Separately, BMSCs are easily accessible, pluripotent and proliferate rapidly as promising candidates for tissue engineering [53–56]. The differentiation of BMSCs depends on culture conditions, concerning of agents, hormones and GFs. Printed BMSC-laden MG scaffolds with CBD-BMP2-collagen microfibers are shown in figure 5(A). BMSCs and CBD-BMP2-collagen microfibers were found to distribute well in the hydrogel network. The viability of BMSCs in the printed scaffolds was analyzed with live/dead cell staining (figure 5(B)), where live cells were stained with calcein-AM and showed green fluorescence, and dead cells were stained with PI and showed red fluorescence. In addition, cell nuclei were stained with DAPI and displayed blue fluorescence. Live/dead cell staining (supporting information figure S1) indicated that the cell viability was 91.8 ± 0.9% after printing. Furthermore, the cell viability at day 7 and day 28 was calculated as 92.1 ± 1.5% and 94.9 ± 1.6%, respectively (figure 5(C)). Notably, the DNA content increased from 34 ± 1 μg mL−1 g to 67 ± 3 μg mL−1 g, which was consistent with cellular proliferation in the printed scaffolds (figure 5(D)).
Figure 5. Printed BMSC-laden methacrylamide gelatin scaffolds with CBD-BMP2-collagen microfibers. (A) Printed hydrogel scaffolds observed under transmission light, scale bar = 50 μm, ▲ shows BMSCs, ● shows CBD-BMP2-collagen microfibers, and the dotted line shows the hydrogel boundary. (B) Live/dead cell staining of printed scaffolds, scale bar = 100 μm, where the live cells show green, the dead cells are red and the nuclei are blue, and the dotted line is the hydrogel boundary. (C) Cell viability of BMSCs in different printed scaffolds on various time points (Day 0, 7, 28). (D) DNA content of BMSCs in different printed scaffolds on various time points (Day 0, 7, 28).
Download figure:
Standard image High-resolution image3.4. BMSCs differentiation into osteocytes in the printed scaffolds
The proliferation and differentiation of sufficient progenitor cells is critical for bone regeneration, which is regulated by GFs of TGF-β and BMP [57–59]. The dose needed to induce bone formation can be greatly reduced once BMP is combined with some appropriate carriers [60]. Furthermore, due to potential adverse effects, it is safer to strictly limit BMP to the wound site [61, 62]. Therefore, we assembled CBD-BMP2-collagen microfibers as differentiation-control modules into BMSC-laden MG scaffolds during 3D bioprinting.
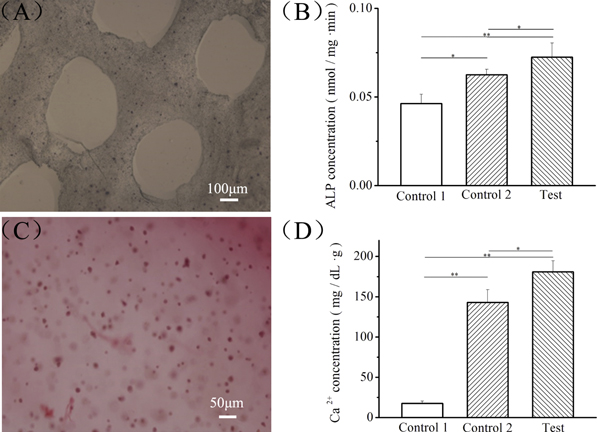
BMSC differentiation into osteocytes in the printed scaffolds was confirmed at gene level using qPCR method (figure 6). As shown in the RT-PCR results, compared with the control 1 and control 2 samples, more gene expression of osteogenic markers, such as ALP, BSP, OCN and COLLA1, could be found in the test samples. The upregulated gene expression confirmed the successful osteogenic differentiation of BMSCs in the printed MG scaffolds with CBD-BMP2-collagen microfibers, leading to a mature osteoblast phenotype [63]. Furthermore, the functional expression in osteogenic differentiation was confirmed using ALP staining (figure 7(A)) and alizarin red staining (figure 7(C)), where ALP staining for osteoblastic markers displays as deep blue and the calcium deposition of osteocytes displays as deep red.
Figure 6. The osteogenic differentiation of BMSCs at gene level in printed methacrylamide gelatin scaffolds with CBD-BMP2-collagen microfibers. The osteogenic markers: ALP, BSP, OCN and COLLA1 were observed using RT-PCR method.
Download figure:
Standard image High-resolution imageFigure 7. Functional expression of differentiated BMSCs in the methacrylamide gelatin scaffolds with CBD-BMP2-collagen microfibers. (A) ALP staining in BMSC-laden printed scaffolds, scale bar = 100 μm. (B) ALP concentration in different samples, *p < 0.05, **p < 0.01. (C) Alizarin red staining BMSC-laden printed scaffolds, scale bar = 100 μm. (D) Ca2+ concentration in different samples, *p < 0.05, **p < 0.01. Control group 1: samples without CBD-BMP2-collagen microfibers in the growth medium; control group 2: samples without microfibers in the osteogenic medium; test group: samples with microfibers in the growth medium comprise the test group.
Download figure:
Standard image High-resolution imageTo further confirm CBD-BMP2-collagen microfibers as differentiation-control modules in printed scaffolds, the differentiation behaviors of BMSCs in the printed scaffolds were compared in three microenvironments: samples without CBD-BMP2-collagen microfibers in the growth medium as control group 1, samples without microfibers in the osteogenic medium as control group 2 and samples with microfibers in the growth medium as the test group. After 14 days in culture, the ALP and Ca2+ concentrations in different samples were compared, respectively. In figure 7(B), the ALP concentration in the test group (0.072 ± 0.0079 nmol mg−1 min−1) is higher than the ALP concentrations of the control groups 1 (0.046 ± 0.0053 nmol mg−1 min−1) and 2 (0.062 ± 0.0031 nmol mg−1 min−1). In figure 7(D), the Ca2+ concentration of the test group (180.86 ± 13.69 mg dL−1 g−1) is higher than the Ca2+ concentrations of control groups 1 (17.57 ± 2.75 mg dL−1 g−1) and 2 (142.84 ± 16.03 mg dL−1 g−1). These results indicated that CBD-BMP2-collagen microfibers could induce BMSC differentiation into osteocytes within 14 days more efficiently than the osteogenic medium. Through this safe and effective approach, not only can the quantity of BMP2 used for BMSC differentiation be reduced, but BMP2 can also be limited to specific printed sites, thus preventing diffusion into the growth medium.
4. Conclusions
We assembled CBD-BMP2-collagen microfibers as differentiation-control modules into BMSC-laden MG scaffolds during 3D bioprinting. The microstructure of the printed scaffolds was able to be tuned with a customized 3D printer. Collagen microfibers were rendered compatible to the printing nozzle and bound CBD-BMP2. BMSCs showed high cell viability (>90%) during printing. CBD-BMP2-collagen microfibers induced BMSC differentiation into osteocytes without soluble GFs. Our studies suggest that these function-control modules are attractive biomaterials with potential applications in 3D bioprinting.
Acknowledgments
The authors acknowledge funding support from the Research Equipment Development Program of the Chinese Academy of Sciences (YZ201351), the National High Technology Research and Development Program of China (863 Program 2012AA020501) and the National Natural Science Foundation of China (51305438), the Ministry of Science and Technology of China (973 Program 2011CB965001) and the 'Strategic Priority Research Program' of the Chinese Academy of Sciences (XDA01030000).