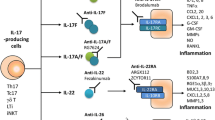
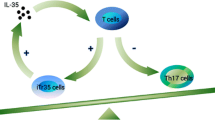
Abstract
The emergence of genetically engineered biological agents opened new prospects in the treatment of autoimmune and inflammatory diseases. Cytokines responsible for regulation of a wide range of processes during development of the normal immune response are among the most successful therapeutic targets. Studies carried out in recent decades and accompanied by rapid development of biotechnology have promoted establishing in detail the role and place of cytokines in autoimmune and inflammatory pathologies. Nevertheless, mechanisms that underlie anti-cytokine therapy are still not fully understood. This review examines the role of such cytokines as TNF, IL-1, and IL-6 in the development of inflammatory processes and the action mechanisms of their inhibitors.
Similar content being viewed by others
References
Carswell, E. A., Old, L. J., Kassel, R. L., Green, S., Fiore, N., and Williamson, B. (1975) An endotoxin serum factor that causes necrosis of tumor, Proc. Natl. Acad. Sci. USA, 72, 3666–3670.
Tracey, D., Klareskog, L., Sasso, E. H., Salfeld, J. G., and Tak, P. P. (2008) Tumor necrosis factor antagonist mechanisms of action: a comprehensive review, Pharmacol. Therap., 117, 244–279.
Black, R. A., Rauch, C. R., Kozlovsky, C. J., Peschon, J. J., Slack, J. L., Wolfson, M. F., Castner, B. J., Stocking, K. L., Reddy, P., Srinivasan, S., Nelson, N., Boiani, N., Schooley, K. A., Gerhart, M., Davis, R., Fitzner, J. N., Johnson, R. S., Paxton, R. J., March, C. J., and Cerretti, D. P. (1997) A metalloproteinase disintegrin that releases tumor-necrosis factor-alpha from cells, Nature, 385, 729–733.
Simmonds, R. E., and Foxwell, B. M. (2008) NF-κB and its relevance to arthritis and inflammation, Rheumatology, 47, 584–590.
Grell, M., Douni, E., Wajant, H., Lohden, M., Clauss, M., Maxeiner, B., Georgopoulus, S., Lesslauer, W., Kollias, G., Pfizenmaier, K., and Scheurich, P. (1995) The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor, Cell, 83, 793–802.
Grell, M., Becke, F. M., Wajant, H., Mannel, D. N., and Scheurich, P. (1998) TNF receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1, Eur. J. Immunol., 28, 257–263.
Carpentier, I., Coornaert, B., and Beyaert, R. (2004) Function and regulation of tumor necrosis factor receptor type 2, Curr. Med. Chem., 11, 2205–2212.
Chen, X., Wu, X., Zhou, Q., Howard, O. M. Z., Netea, M. G., and Oppenheim, J. J. (2013) TNFR2 is critical for stabilization of the CD4+FoxP3+ regulatory T cell phenotype in the inflammatory environment, J. Immunol., 190, 1076–1084.
Redl, H., Schlag, G., Adolf, G. R., Natmessing, B., and Davies, J. (1995) Tumor necrosis factor (TNF)-dependent shedding of the p55 TNF receptor in a baboon model of bacteremia, Infect. Immun., 63, 297–300.
Aderka, D., Engelmann, H., Maor, Y., Brakebusch, C., and Wallach, D. (1992) Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors, J. Exp. Med., 175, 323–329.
Keeton, R., Allie, N., Dambuza, I., Abel, B., Hsu, N.-J., Sebesho, B., Randall, P., Burger, P., Fick, E., Quesniaux, V. F. J., Ryffel, B., and Jacobs, M. (2014) Soluble TNFRp75 regulates host protective immunity against Mycobacterium tuberculosis, J. Clin. Invest., 124, 1537–1551.
Schett, G. (2009) Osteoimmunology in rheumatic diseases, Arthr. Res. Therap., 11, 210–216.
Abu-Amer, Y., Erdmann, J., Kollias, G., Alexopoulou, L., Ross, P., and Teitelbaum, S. L. (2000) Tumor necrosis factor receptors types 1 and 2 differentially regulate osteoclastogenesis, J. Biol. Chem., 275, 27307–27310.
Requeiro, M., Kip, K. E., Baidoo, L., Swoqer, J. M., and Schraut, W. (2014) Postoperative therapy with infliximab prevents long-term Crohn’s disease recurrence, Clin. Gastroenterol. Hepatol., 12, 1494–1502.
Verazza, S., Negro, G., Marafon, D., Consolaro, A., Martini, A., and Ravelli, A. (2013) Possible discontinuation of therapies after clinical remission in juvenile idiopathic arthritis, Clin. Exp. Rheumatol., 31, S98–101.
Huang, Z., Yang, B., Shi, Y., Cai, B., Li, Y., Feng, W., Fu, Y., Luo, L., and Wang, L. (2012) Anti-TNF-α therapy improves Treg and suppresses Teff in patients with rheumatoid arthritis, Cell. Immunol., 279, 25–29.
Tanaka, Y., Hirata, S., Kubo, S., Fukuyo, S., Hanami, K., Sawamukai, N., Nakano, K., Nakayamada, S., Yamaoka, K., Sawamura, F., and Saito, K. (2013) Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1 year outcome of the HONOR study, Ann. Rheum. Dis., 2013, Nov 28, doi: 10.1136/annrheumdis-2013-204016.
Targan, S. R., Hanauer, S. B., Van Deventer, S. J., Mayer, L., Present, D. H., Braakman, T., DeWoody, K. L., Schaible, T. F., and Rutgeerts, P. J. (1997) A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s disease cA2 study group, N. Engl. J. Med., 337, 1029–1035.
Van Dullemen, H. M., Van Deventer, S. J., Hommes, D. W., Bijl, H. A., Jansen, J., Tytgat, G. N., and Woody, J. (1995) Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2), Gastroenterology, 109, 129–135.
Braun, J., Brandt, J., Listing, J., Zink, A., Alten, R., Golder, W., Gromnica-Ihle, E., Kellner, H., Krause, H., Schneider, M., Sorensen, H., Zeidler, H., Thriene, W., and Sieper, J. (2002) Treatment of active ankylosing spondylitis with infliximab: a randomized controlled multicentre trial, Lancet, 359, 1187–1193.
Kruithof, E., Van den Bosch, F., Baeten, D., Herssens, A., De Keyser, F., Mielants, H., and Veys, E. M. (2002) Repeated infusion of infliximab, a chimeric anti-TNF-alpha monoclonal antibody, in patients with active spondyloarthropathy: one years follow up, Ann. Rheum. Dis., 61, 207–212.
Chaudhari, U., Romano, P., Mulcahy, L. D., Dooley, L. T., Baker, D. G., and Gottlieb, A. B. (2001) Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: randomized trial, Lancet, 357, 1842–1847.
Knight, D. M., Trinh, H., Le, J., Siegel, S., Shealy, D., McDonough, M., Scallon, B., Moore, M. A., Vilcek, J., and Daddona, P. (1993) Construction and initial characterization of a mouse-human chimeric anti-TNF antibody, Mol. Immunol., 30, 1143–1453.
Scallon, B. J., Moore, M. A., Trinh, H., Knight, D. M., and Ghrayeb, J. (1995) Chimeric anti-TNF-alpha monoclonal antibody cA2 binds recombinant transmembrane TNF-alpha and activates immune effector functions, Cytokine, 7, 251–259.
Kruglov, A. A., Grivennikov, S. I., Kuprash, D. V., Winsauer, C., Prepens, S., Seleznik, G. M., Ebert, G., Littman, D. R., Heikenwalder, M., Tumanov, A. V., and Nedospasov, S. A. (2013) Nonredundant function of soluble LTa3 produced by innate lymphoid cells in intestinal homeostasis, Science, 342, 1243–1246.
Lukina, G. V., and Sigidin, Ya. A. (2008) Safety of therapy with adalimumab, Nauch. Prakt. Revmatol., 2, 60–63.
Sigidin, Ya. A., and Lukina, G. V. (2008) Adalimumab in therapy of early rheumatoid arthritis, Nauch. Prakt. Revmatol., 2, 56–59.
Kay, J., and Rahman, U. (2009) Golimumab: a novel human anti-TNF-α monoclonal antibody for the treatment of rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis, Core Evidence, 4, 159–170.
Lukina, G. V., and Sigidin, Ya. A. (2012) Certolizumab in therapy of rheumatoid arthritis, Sovrem. Revmatol., 2, 44–49.
Shealy, D., Cai, A., Staquet, K., Baker, A., Lacy, E. R., Johns, L., Vafa, O., Gunn III, G., Tam, S., Sague, S., Wang, D., Brigham-Burke, M., Dalmonte, P., Emmell, E., Pikounis, B., Bugelski, P. J., Zhou, H., Scallon, B., and Giles-Komar, J. (2010) Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor α, MAbs, 2, 428–439.
Schaible, H.-G., Von Anchet, G. S., Boettger, M. K., Brauer, R., Gajda, M., Richter, F., Hensellek, S., Brenn, D., and Natura, G. (2010) The role of proinflammatory cytokines in the generation and maintenance of joint pain, Ann. NY Acad. Sci., 1193, 60–69.
Notley, C. A., Inglis, J. J., Alzabin, S., McCann, F. E., McNamee, K. E., and Williams, R. O. (2008) Blockade of tumor necrosis factor in collagen-induced arthritis reveals a novel immunoregulatory pathway for Th1 and Th17 cells, J. Exp. Med., 205, 2491–2497.
Szalay, B., Vasarhelyi, B., Cseh, A., Tulassay, T., Deak, M., Kovacs, L., and Balog, A. (2013) The impact of conventional DMARD and biological therapies on CD4+ cell subsets in rheumatoid arthritis: a follow up study, Clin. Rheumatol., 33, 175–185.
Evans, H. G., Roostalu, U., Walter, G. J., Gullick, N. J., Frederiksen, K. S., Roberts, C. A., Sumner, J., Baeten, D. L., Gerwien, J. G., Cope, A. P., Geissmann, F., Kirkham, B. W., and Taams, L. S. (2014) TNF-α blockade induces IL-10 expression in human CD4+ T cells, Nat. Commun., 5, 3199–3211.
Anolik, J. H., Ravikumar, R., Barnard, J., Owen, T., Almudevar, A., Milner, E. C., Miller, C. H., Dutcher, P. O., Hadley, J. A., and Sanz, I. (2008) Cutting edge: anti-tumor necrosis factor therapy in rheumatoid arthritis inhibits memory B lymphocytes via effects on lymphoid germinal centers and follicular dendritic cell networks, J. Immunol., 180, 688–692.
Valencia, X., Stephens, G., Goldbach-Mansky, R., Wilson, M., Shevach, E. M., and Lipsky, P. E. (2006) TNF down-modulates the function of human CD4+ CD25hi T-regulatory cells, Blood, 108, 253–261.
Nie, H., Zheng, Y., Li, R., Cuo, T. B., He, D., Fang, L., Liu, X., Xiao, L., Chen, X., Wan, B., Chin, Y. E., and Zhang, J. Z. (2013) Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis, Nat. Med., 19, 322–328.
Dinarello, C. A. (1994) The interleukin-1 family: 10 years of discovery, FASEB J., 8, 1314–1325.
Arend, W. P., Malyak, M., Guthridge, C. J., and Gabay, C. (1998) Interleukin-1 receptor antagonist: role in biology, Ann. Rev. Immunol., 16, 27–55.
Magne, D., Palmer, G., Barton, J. L., Mezin, F., Talabot-Ayer, D., Bas, S., Duffy, T., Noger, M., Guerne, P.-A., Nicklin, M. J. H., and Gabay, C. (2006) The new IL-1 family member IL-1F8 stimulates production of inflammatory mediators by synovial fibroblast and articular, Arthr. Res. Ther., 8, R80.
Kumar, S., McDonnell, P. C., Lehr, R., Tierney, L., Tzimas, M. N., Griswold, D. E., Capper, E. A., Tal-Singer, R., Wells, G. I., Doyle, M. L., and Young, P. R. (2000) Identification and initial characterization of four novel members of the interleukin-1 family, J. Biol. Chem., 275, 10308–10314.
Smith, D. E., Renshaw, B. R., Ketchem, R. R., Kubin, M., Garka, K. E., and Sims, J. E. (2001) Four new members expand the interleukin-1 family, J. Biol. Chem., 275, 1169–1175.
Dinarello, C. A. (1996) Biologic basis for interleukin-1 in disease, Blood, 87, 2095–2147.
Sims, J. E., and Smith, D. E. (2010) The IL-1 family: regulators of immunity, Nat. Rev. Immunol., 10, 89–102.
O’Neill, L. A. J. (2008) The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress, Immunol. Rev., 226, 10–18.
Garlanda, C., Dinarello, C. A., and Mantovani, A. (2013) The interleukin-1 family: back to the future, Immunity, 39, 1003–1018.
Martinon, F., Mayor, A., and Tschopp, J. (2009) The inflammasomes: guardians of the body, Ann. Rev. Immunol., 27, 229–269.
Gross, O., Yazdi, A. S., Thomas, C. J., Masin, M., Heinz, L. X., Guarda, G., Quadroni, M., Drexler, S. K., and Tschopp, J. (2012) Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1, Immunity, 36, 388–400.
Colotta, F., Re, F., Muzio, M., Bertini, R., Polentarutti, N., Sironi, M., Giri, J. G., Dower, S. K., Sims, J. E., and Mantovani, A. (1993) Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4, Science, 261, 472–475.
Colotta, F., Dower, S. K., Sims, J. E., and Mantovani, A. (1994) The type II “decoy” receptor: a novel regulatory pathway for interleukin 1, Immunol. Today, 15, 562–528.
Penton-Rol, G., Orlando, S., Polentarytti, N., Bernasconi, S., Muzio, M., Introna, M., and Mantovani, A. (1999) Bacterial lipopolysaccharide causes rapid shedding, followed by inhibition of mRNA expression, of the IL-1 type II receptor, with concomitant up-regulation of the type I receptor and induction of incompletely spliced transcript, J. Immunol., 162, 2931–2938.
Barksby, H. E., Lea, S. R., and Preshaw, P. M. (2007) The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders, Clin. Exp. Immunol., 149, 217–225.
Smith, D. E., Hanna, R., Friend, D., Moore, H., Chen, H., Farese, A. M., MacVittie, T. J., Virca, G. D., and Sims, J. E. (2003) The soluble form of IL-1 receptor accessory protein enhances the ability of soluble type II IL-1 receptor to inhibit IL-1 action, Immunity, 18, 87–96.
Donath, M. Y. (2014) Targeting inflammation in the treatment of type 2 diabetes: time to start, Nature Rev. Drug Discov., 13, 465–476.
Dinarello, C. A., and van der Meer, J. W. M. (2013) Treating inflammation by blocking interleukin-1 in humans, Semin. Immunol., 25, 469–484.
Khanna, P., Gladue, H. S., Singh, M. K., FitzGerald, D., Bae, S., Prakash, S., Kaldas, M., Gogia, M., Berrocal, V., Townsend, W., Terkeltaub, R., and Khanna, D. (2014) Treatment of acute gout: a systematic review, Sem. Arthritis Rheum., 44, 31–38.
Sterba, G., and Sterba, Y. (2013) Controlling inflammation. Contemporary treatment for autoinflammatory diseases and syndromes, Dermatol. Clin., 31, 507–511.
Gabay, C., and Arend, W. P. (1998) Treatment of rheumatoid arthritis with IL-1 inhibitors, Springer Semin. Immunopathol., 20, 229–246.
Bunning, R. A., Richardson, H. J., Crawford, A., Skiodt, H., Hughes, D., Evans, D. B., Gowen, M., Dobson, P. R., Brown, B. L., and Russell, R. (1986) The effect of interleukin-1 on connective tissue metabolism and its relevance to arthritis, Agents Actions Suppl., 18, 131–152.
Volin, M. V., Shah, M. R., Tokuhira, M., Haines, G. K., Woods, J. M., and Koch, A. E. (1998) RANTES expression and contribution to monocyte chemotaxis in arthritis, Clin. Immunol. Immunopathol., 89, 44–53.
Nakatsuka, K., Tanaka, Y., Hubscher, S., Abe, M., Wake, A., Saito, K., Morimoto, I., and Eto, S. (1997) Rheumatoid synovial fibroblasts are stimulated by the cellular adhesion to T cells through lymphocyte function associated antigen-1/intercellular adhesion molecule-1, J. Rheumatol., 24, 458–464.
Garcia-Hernandez, M. H., Gonzalez-Amaro, R., and Portales-Perez, D. P. (2014) Specific therapy to regulate inflammation in rheumatoid arthritis: molecular aspects, Immunotherapy, 6, 623–636.
Arend, W. P., and Gabay, C. (2004) Cytokines in the rheumatic diseases, Rheum. Dis. Clin. N. Am., 30, 41–67.
Chandrasekhar, S., and Phadke, K. (1988) Interleukin-1-induced alterations in proteoglycan metabolism and matrix assembly, Arch. Biochem. Biophys., 265, 294–301.
Murata, M., Bonassar, L. J., Wright, M., Mankin, H. J., and Towle, C. A. (2003) A role for the interleukin-1 receptor in the pathway linking static mechanical compression to decreased proteoglycan synthesis in surface articular cartilage, Arch. Biochem. Biophys., 413, 229–235.
Ikeda, S., Saijo, S., Murayama, M. A., Shimizu, K., Akitsu, A., and Iwakura, Y. (2014) Excess IL-1 signaling enhances the development of Th17 cells by down-regulating TGF-β-induced Foxp3 expression, J. Immunol., 192, 1449–1458.
Brennan, F. M., and McInnes, I. B. (2008) Evidence that cytokines play a role in rheumatoid arthritis, J. Clin. Invest., 118, 3537–3545.
Opal, S. M., Fisher, C. J., Dhainaut, J. F., Vincent, J. L., Brase, R., Lowry, S. F., Sadoff, J. C., Slotman, G. J., Levy, H., Balk, R. A., Shelly, M. P., Pribble, J. P., LaBrecque, J. F., Lookabaugh, J., Donovan, H., Dubin, H., Baughman, R., Noeman, J., DeMaria, E., Matzek, K., Abraham, E., and Seneff, M. (1997) Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group, Crit. Care Med., 25, 1115–1124.
Fisher, C. J., Dhainaut, J. F., Opal, S. M., Pribble, J. P., Balk, R. A., Slotman, G. J., Iberti, T. J., Rackow, E. C., Shapiro, M., and Greenman, R. L. (1994) Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group, JAMA, 271, 1836–1843.
Hirano, T., Yasukawa, K., Harada, H., Taga, T., Watanabe, Y., Matsuda, T., Kashiwamura, S., Nakajima, K., Koyama, K., Iwamatsu, A., Tsunasawa, S., Sakiyama, F., Matsu, H., Takahara, Y., Taniguchi, T., and Kishimoto, T. (1986) Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin, Nature, 324, 73–76.
Rose-John, S. (2012) IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6, Int. J. Biol. Sci., 8, 1237–1247.
White, U. A., and Stephens, J. M. (2011) The gp130 receptor cytokine family: regulators of adipocyte development and function, Curr. Pharm. Des., 17, 340–346.
Kishimoto, J., Akira, S., and Taga, T. (1992) IL-6 receptor mechanism of signal transduction, Int. J. Immunopharmacol., 14, 431–438.
Heinrich, P. C., Behrmann, I., Muller-Newen, G., Schaper, F., and Graeve, L. (1998) Interleukin-6-type cytokine signaling through the gp130/JAK/STAT pathway, Biochem. J., 334, 297–314.
Mullberg, J., Schooltink, H., Stoyan, T., Gunther, M., Graeve, L., Buse, G., Mackiewicz, A., Heinrich, P. C., and Rose-John, S. (1993) The soluble interleukin-6 receptor is generated by shedding, Eur. J. Immunol., 23, 473–480.
Hurst, S. M., Wilkinson, T. S., McLoughlin, R. M., Jones, S., Horiuchi, S., Yamamoto, N., Rose-John, S., Fuller, G. M., Topley, N., and Jones, S. A. (2001) IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation, Immunity, 14, 706–714.
Horiuchi, S., Koyanagi, Y., Miyamoto, H., Tanaka, Y., and Waki, M. (1994) Soluble interleukin-6 receptors released from T cell or granulocyte/macrophage cell lines and human peripheral blood mononuclear cells are generated through an alternative splicing mechanism, Eur. J. Immunol., 24, 1945–1948.
Briso, E. M., Dienz, O., and Rincon, M. (2008) Cutting edge: soluble IL-6R is produced by IL-6R ectodomain shedding activates CD4 T cell, J. Immunol., 180, 7102–7106.
Lotz, M., Jirik, F., Kabouridis, P., Tsoukas, C., Hirano, T., Kishimoto, T., and Carson, D. A. (1988) B cell stimulating factor 2/interleukin 6 is a costimulant for human thymocytes and T lymphocytes, J. Exp. Med., 167, 1253–1258.
Sehgal, P. B. (1990) Interleukin-6: molecular pathophysiology, J. Invest. Dermatol., 94, 2S–6S.
Hirano, T. (1998) Interleukin 6 and its receptor: ten years later, Int. Rev. Immunol., 16, 249–284.
Striz, I., Brabcova, E., Kolesar, L., and Sekerkova, A. (2014) Cytokine networking of innate immunity cells: a potential target of therapy, Clin. Sci., 126, 593–612.
Marz, P., Cheng, J.-G., Gadient, R. A., Patterson, P. H., Stoyan, T., Otten, U., and Rose-John, S. (1998) Sympathetic neurons can produce and respond to interleukin 6, Proc. Natl. Acad. Sci. USA, 95, 3251–3256.
Streit, W. J., Hurley, S. D., McGraw, T. S., and Semple-Rowland, S. L. (2000) Comparative evaluation of cytokine profiles and reactive gliosis supports a critical role from interleukin-6 in neuroglia signaling during regeneration, J. Neurosci. Res., 61, 10–20.
Wallenius, V., Wallenius, K., Ahren, B., Rudling, M., Carlsten, H., Dickson, S. L., Ohlsson, C., and Jansson, J. O. (2002) Interleukin-6-deficient mice develop mature-onset obesity, Nature Med., 8, 75–79.
Erta, M., Quintana, A., and Hidalgo, J. (2012) Interleukin-6, major cytokine in the central nervous system, Int. J. Biol. Sci., 8, 1254–1266.
Scheller, J., Garbers, C., and Rose-John, S. (2014) Interleukin-6: from basic to selective blockade of pro-inflammatory activities, Semin. Immunol., 26, 2–12.
Tanaka, T., and Kishimoto, T. (2012) Targeting interleukin-6: all the way to treat autoimmune and inflammatory disease, Int. J. Biol. Sci., 8, 1227–1236.
Kimura, A., and Kishimoto, T. (2010) IL-6: regulator of Treg/Th17 balance, Eur. J. Immunol., 40, 1830–1835.
Samson, M., Audia, S., Janikashvili, N., Ciudad, M., Trad, M., Fraszczak, J., Ornetti, P., Maillefert, J. F., Miossec, P., and Bonnotte, B. (2012) Brief report: inhibition of interleukin-6 function corrects TH17/Treg cell imbalance in patients with rheumatoid arthritis, Arthritis Rheum., 64, 2499–24503.
Nishida, S., Haqihara, K., Shima, Y., Kawai, M., Kuwahara, Y., Arimitsu, J., Hirano, T., Narazaki, M., Ogata, A., Yoshizaki, K., Kawase, I., Kishimoto, T., and Tanaka, T. (2009) Rapid improvement of AA amyloidosis with humanized anti-interleukin 6 receptor antibody treatment, Ann. Rheum. Dis., 68, 1235–1236.
Roll, P., Muhammad, K., Schumann, M., Kleinert, S., Einsele, H., Dorner, T., and Tony, H. P. (2011) In vivo effect of the anti-interleukin-6 receptor tocilizumab on the B cell compartment, Arthritis Rheum., 63, 1255–1264.
Thiolat, A., Semerano, L., Pers, Y. M., Biton, J., Lemeiter, D., Portales, P., Quentin, J., Jorgensen, C., Decker, P., Boissier, M. C., Louis-Plence, P., and Bessis, N. (2014) Interleukin-6 receptor blockade enhances CD39+ regulatory T cell development in rheumatoid arthritis and in experimental arthritis, Arthritis Rheum., 66, 273–283.
Tanaka, T., Hishitani, Y., and Ogata, A. (2014) Monoclonal antibodies in rheumatoid arthritis: comparative effectiveness of tocilizumab with tumor necrosis factor inhibitors, Biologics: Targets Therapy, 8, 141–153.
Tanaka, Y., and Mola, E. M. (2014) IL-6 targeting compared to TNF targeting in rheumatoid arthritis: studies of olokizumab, sarilumab and sirukumab, Ann. Rheum. Dis., 73, 1595–1597.
Kallen, K.-J. (2002) The role of trans-signaling via the agonistic soluble IL-6 receptor in human disease, Biochim. Biophys. Acta, 1592, 323–343.
Chalaris, A., Schmidt-Arras, D., Yamamoto, K., and Rose-John, S. (2012) Interleukin-6 trans-signaling and colonic cancer associated with inflammatory bowel disease, Digest. Dis., 30, 492–499.
Barkhausen, T., Tschernig, T., Rosenstiel, T., van Griensven, M., Vonberg, R.-P., Dorsch, M., Mueller-Heine, A., Chalaris, A., Scheller, J., Rose-John, S., Seegert, D., Krettek, C., and Waetzig, G. (2011) Selective blockade of interleukin-6 trans-signaling improves survival in a murine polymicrobial sepsis model, Crit. Care Med., 39, 1407–1413.
Nowell, M. A., Williams, A. S., Carty, S. A., Scheller, J., Hayes, A. J., Jones, G. W., Richards, P. J., Slinn, S., Ernst, M., Jemkins, B. J., Topley, N., Rose-John, S., and Jones, S. A. (2009) Therapeutic targeting of IL-6 trans-signaling counteracts STAT3 control of experimental inflammatory arthritis, J. Immunol., 182, 613–622.
Atreys, R., Mudter, J., Finotto, S., Mullberg, J., Jostock, T., Wirtz, S., Schutz, M., Bartsch, B., Holtmann, M., Becker, C., Strand, D., Czaja, J., Schlaak, J. F., Lehr, Y. A., Autschbach, F., Schurmann, G., Nishimoto, N., Yoshizaki, K., Ito, H., Kishimoto, T., Galle, P. R., Rose-John, S., and Neurath, M. F. (2000) Blockade of interleukin 6 trans-signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn’s disease and experimental colitis in vivo, Nature Med., 6, 583–588.
Ramiro, S., Gaujoux-Viala, C., Nam, J. L., Smolen, J. S., Buch, M., Gossec, L., Van Der Heijde, D., Winthrop, K., and Landewe, R. (2014) Safety of synthetic and biological DMARDs: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis, Ann. Rheum. Dis., 73, 529–535.
Rubbert-Roth, A. (2012) Assessing the safety of biologic agents in patients with rheumatoid arthritis, Rheumatology, 51, 38–47.
Kaltsonoudis, E., Voulgari, P. V., Konitsiois, S., and Drosos, A. A. (2014) Demyelination and other neurological adverse events after anti-TNF therapy, Autoimmun. Rev., 13, 54–58.
Prinz, J. C. (2011) Autoimmune-like syndromes during TNF blockade: does infection have a role? Nat. Rev. Rheumatol., 7, 429–434.
Winsauer, C., Kruglov, A. A., Chashchina, A. A., Drutskaya, M. S., and Nedospasov, S. A. (2014) Cellular sources of pathogenic and protective TNF and experimental strategies on utilization of TNF humanized mice, Cytokine Growth Factor Rev., 25, 115–123.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Original Russian Text © I. V. Astrakhantseva, G. A. Efimov, M. S. Drutskaya, A. A. Kruglov, S. A. Nedospasov, 2014, published in Biokhimiya, 2014, Vol. 79, No. 12, pp. 1605–1616.
Rights and permissions
About this article
Cite this article
Astrakhantseva, I.V., Efimov, G.A., Drutskaya, M.S. et al. Modern anti-cytokine therapy of autoimmune diseases. Biochemistry Moscow 79, 1308–1321 (2014). https://doi.org/10.1134/S0006297914120049
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297914120049