Abstract
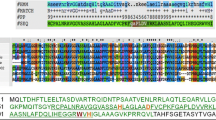
Bacteriophage endolysins degrading bacterial cell walls are prospective enzymes for therapy of bacterial infections. The genome of the giant bacteriophage phiKZ of Pseudomonas aeruginosa encodes two endolysins, gene products (g.p.) 144 and 181, which are homologous to lytic transglycosylases. Gene 144 encoding a 260 amino acid residue protein was cloned into the plasmid expression vector. Recombinant g.p. 144 purified from Escherichia coli effectively degrades chloroform-treated P. aeruginosa cell walls. The protein has predominantly α-helical conformation and exists in solution in stoichiometric monomer: dimer: trimer equilibrium. Antibodies against the protein bind the phage particle. This demonstrates that g.p. 144 is a structural component of the phiKZ particle, presumably, a phage tail.
Similar content being viewed by others
Abbreviations
- PMSF:
-
o-phenylmethylsulfonyl fluoride
- IPTG:
-
isopropyl β-D-thiogalactopyranoside
- PCR:
-
polymerase chain reaction; g.p.) gene product
- bp:
-
nucleotide base pairs
References
Hanlon, G. W., Denyer, S. P., Olliff, C. J., and Ibrahim, L. J. (2001) Appl. Environ. Microbiol., 67, 2746–2753.
Krylov, V. N. (2001) Genetika, 37, 869–878.
Nelson, D., Loomis, L., and Fischetti, V. A. (2001) Proc. Natl. Acad. Sci. USA, 98, 4107–4112.
Young, R. (2002) J. Bacteriol., 184, 2572–2575.
Bernhardt, T. G., Wang, I. N., Struck, D. K., and Young, R. (2002) Res. Microbiol., 153, 493–501.
Young, R., Wang, I.-N., and Roof, W. D. (2000) Trends Microbiol., 8, 4974–4984.
Schuch, R., Nelson, D., and Fischetti, V.A. (2002) Nature, 418, 884–889.
Jado, I., Lopez, R., Garcia, E., Fenoll, A., Casal, J., and Garcia, P. (2003) J. Antimicrob. Chemother., 52, 967–973.
Giamarellou, H. (2000) Int. J. Antimicrob. Agents, 16, 103–106.
Mesyanzhinov, V. V., Robben, J., Grymonprez, B., Kostyuchenko, V. A., Bourkaltseva, M. V., Sykilinda, N. N., Krylov, V. N., and Volckaert, G. (2002) J. Mol. Biol., 317, 1–19.
Bourkaltseva, M. V., Krylov, V. N., Pleteneva, E. A., Shaburova, O. V., Krylov, S. V., Volckaert, G., Sykilinda, N. N., Kurochkina, L. P., and Mesyanzhinov, V. V. (2002) Genetika, 38, 1470–1479.
Lavigne, R., Burkal’tseva, M. V., Robben, J., Sykilinda, N. N., Kurochkina, L. P., Grymonprez, B., Jonckx, B., Krylov, V. N., Mesyanzhinov, V. V., and Volckaert, G. (2003) Virology, 312, 49–59.
Wang, P. W., Chu, L., and Guttman, D. S. (2004) J. Bacteriol., 186, 400–410.
Provencher, S. W., and Glockner, J. (1981) Biochemistry, 20, 33–37.
Venyaminov, S. Yu., Baikalov, I. A., Shen, Z. M., Wu, C.-S. C., and Yang, J. T. (1993) Analyt. Biochem., 214, 17–24.
Sreerama, N., and Woody, R. W. (1993) Analyt. Biochem., 209, 32–44.
Laemmli, U. K. (1970) Nature, 227, 680–685.
Towbin, J., Staehelen, T., and Gordon, J. (1979) Proc. Natl. Acad. Sci. USA, 76, 4350–4354.
Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D. J. (1997) Nucleic Acids Res., 25, 3389–3402.
Marchler-Bauer, A., Anderson, J. B., DeWeese-Scott, C., Fedorova, N. D., Geer, L. Y., He, S., Hurwitz, D. I., Jackson, J. D., Jacobs, A. R., Lanczycki, C. J., Liebert, C. A., Liu, C., Madej, T., Marchler, G. H., Mazumder, R., Nikolskaya, A. N., Panchenko, A. R., Rao, B. S., Shoemaker, B. A., Simonyan, V., Song, J. S., Thiessen, P. A., Vasudevan, S., Wang, Y., Yamashita, R. A., Yin, J. J., and Bryant, S. H. (2003) Nucleic Acids Res., 31, 383–387.
Holtje, J. V., Mirelman, D., Sharon, N., and Schwarz, U. (1975) J. Bacteriol., 124, 1067–1076.
Vocadlo, D. J., Davies, G. J., Laine, R., and Withers, S. G. (2001) Nature, 412, 835–838.
Van Asselt, E. J., Kalk, K. H., and Dijkstra, B. W. (2000) Biochemistry, 39, 1924–1934.
Tews, I., Perrakis, A., Oppenheim, A., Dauter, Z., Wilson, K. S., and Vorgias, C. E. (1996) Nat. Struct. Biol., 3, 638–648.
Koraimann, G. (2003) Cell Mol. Life Sci., 5, 2371–2388.
Kelley, L. A., MacCallum, R. M., and Sternberg, M. J. E. (2000) J. Mol. Biol., 299, 499–520.
Van Asselt, E. J., Perrakis, A., Kalk, K. H., Lamzin, V. S., and Dijkstra, B. W. (1998) Acta Crystallogr. D. Biol. Crystallogr., 54, 58–73.
Reid, C. W., Blackburn, N. T., Legaree, B. A., Auzanneau, F.-I., and Clarke, A. J. (2004) FEBS Lett., 574, 73–79.
Rydman, P. S., and Bamford, D. H. (2002) J. Bacteriol., 184, 104–110.
Monzingo, A. F., Marcotte, E. M., Hart, P. J., and Robertus, J. D. (1996) Nat. Struct. Biol., 3, 133–140.
Dideberg, O., Charlier, P., Dive, G., Joris, B., Frere, J. M., and Ghuysen, J. M. (1982) Nature, 299, 469–470.
Kanamaru, S., Leiman, P. G., Kostyuchenko, V. A., Chipman, P. R., Mesyanzinov, V. V., Arisaka, F., and Rossmann, M. G. (2002) Nature, 415, 553–557.
Verheust, C., Fornelos, N., and Mahillon, J. (2004) FEMS Microbiol. Lett., 237, 289–295.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2006, Vol. 71, No. 3, pp. 379–385.
Rights and permissions
About this article
Cite this article
Miroshnikov, K.A., Faizullina, N.M., Sykilinda, N.N. et al. Properties of the endolytic transglycosylase encoded by gene 144 of Pseudomonas aeruginosa bacteriophage phiKZ. Biochemistry (Moscow) 71, 300–305 (2006). https://doi.org/10.1134/S0006297906030102
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1134/S0006297906030102