Abstract
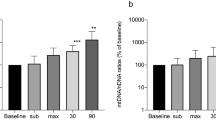
Changes in the level of blood cell-free circulating mitochondrial DNA were examined during experimental adrenaline-induced myocardial injury in rats. The amount of mitochondrial DNA in the blood was significantly elevated at 48 and 72 h after subcutaneous injection of adrenaline solution, and it was accompanied by development of multiple smallfocal myocardial ischemia. This suggests that the measured level of blood cell-free circulating mitochondrial DNA might be used as a biomarker of acute myocardial ischemia.
Similar content being viewed by others
References
Thygesen, K., Alpert, J. S., Jaffe, A. S., Simoons, M. L., Chaitman, B. R., and White, H. D. (2012) Writing group on behalf of the joint ESC/ACCF/AHA/WHF task force for the universal definition of myocardial infarction. Third universal definition of myocardial infarction, J. Am. Coll. Cardiol., 60, 1581–1598.
Seidlmayer, L. K., Juettner, V. V., Kettlewell, S., Pavlov, E. V., Blatter, L. A., and Dedkova, E. N. (2015) Distinct mPTP activation mechanisms in ischemia-reperfusion: contributions of Ca2+, ROS, pH, and inorganic polyphos-phate, Cardiovasc. Res., 106, 237–248.
Gedik, N., Heusch, G., and Skyschally, A. (2013) Infarct size reduction by cyclosporine A at reperfusion involves inhibition of the mitochondrial permeability transition pore but does not improve mitochondrial respiration, Arch. Med. Sci., 9, 968–975.
Kalogeris, T., Bao, Y., and Korthuis, R. J. (2014) Mitochondrial reactive oxygen species: a double-edged sword in ischemia/reperfusion vs preconditioning, Redox Biol., 2, 702–714.
Green, D. R., and Kroemer, G. (2004) The pathophysiolo-gy of mitochondrial cell death, Scienc., 305, 626–662.
Patrushev, M., Kasymov, V., Patrusheva, V., Ushakova, T., Gogvadze, V., and Gaziev, A. (2004) Mitochondrial perme-ability transition triggers the release of mtDNA fragments, Cell Mol. Life Sci., 61, 3100–3103.
Patrushev, M., Kasymov, V., Patrusheva, V., Ushakova, T., Gogvadze, V., and Gaziev, A. I. (2006) Release of mito-chondrial DNA fragments from brain mitochondria of irra-diated mice, Mitochondrio., 6, 43–47.
Garcia, N., and Chavez, E. (2007) Mitochondrial DNA fragments released through the permeability transition pore correspond to specific gene size, Life Sci., 81, 1160–1166.
Chang, C. P., Chia, R. H., Wu, T. L., Tsao, K. C., Sun, C. F., and Wu, J. T. (2003) Elevated cell-free serum DNA detected in patients with myocardial infarction, Clin. Chim. Act., 327, 95–101.
Ellinger, J., Muller, S. C., Wernert, N., von Ruecker, A., and Bastian, P. J. (2008) Mitochondrial DNA in serum of patients with prostate cancer: a predictor of biochemical recurrence after prostatectomy, BJU Int., 102, 628–632.
Nakahira, K., Kyung, S. Y., Rogers, A. J., Gazourian, L., Youn, S., Massaro, A. F., Quintana, C., Osorio, J. C., Wang, Z., Zhao, Y., Lawler, L. A., Christie, J. D., Meyer, N. J., Mc Causland, F. R., Waikar, S. S., Waxman, A. B., Chung, R. T., Bueno, R., Rosas, I. O., Fredenburgh, L. E., Baron, R. M., Christiani, D. C., Hunninghake, G. M., and Choi, A. M. (2013) Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation, PLoS Med., 10, e1001577.
Sun, S., Sursal, T., Adibnia, Y., Zhao, C., Zheng, Y., Li, H., Otterbein, L. E., Hauser, C. J., and Itagaki, K. (2013) Mitochondrial DAMPs increase endothelial permeability through neutrophil dependent and independent pathways, PLoS One, 8, e59989.
Zhang, Q., Itagaki, K., and Hauser, C. J. (2010) Mitochondrial DNA is released by shock and activates neu-trophils via p38 map kinase, Shoc., 34, 55–59.
Zhang, Q., Raoof, M., Chen, Y., Sumi, Y., Sursal, T., Junger, W., Brohi, K., Itagaki, K., and Hauser, C. J. (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury, Natur., 464, 104–107.
Wang, L., Xie, L., Zhang, Q., Cai, X., Tang, Y., Wang, L., Hang, T., Liu, J., and Gong, J. (2015) Plasma nuclear and mitochondrial DNA levels in acute myocardial infarction patients, Coron. Artery Dis., 26, 296–300.
Bliksoen, M., Mariero, L. H., Ohm, I. K., Haugen, F., Yndestad, A., Solheim, S., Seljeflot, I., Ranheim, T., Andersen, G. O., Aukrust, P., Valen, G., and Vinge, L. E. (2012) Increased circulating mitochondrial DNA after myocardial infarction, Int. J. Cardiol., 158, 132–134.
Arnalich, F., Codoceo, R., Lopez-Collazo, E., and Montiel, C. (2012) Circulating cell-free mitochondrial DNA: a better early prognostic marker in patients with out-of-hospital cardiac arrest, Resuscitatio., 83, 162–163.
Mallov, S., and Gilmour, R. F. (1977) Inhibition of epi-nephrine-induced myocardial necrosis in rats by adminis-tration of single doses of ethanol, Drug Alcohol Depend., 2, 397–407.
Chiu, R. W., Chan, L. Y., Lam, N. Y., Tsui, N. B., Ng, E. K., Rainer, T. H., and Lo, Y. M. (2003) Quantitative analy-sis of circulating mitochondrial DNA in plasma, Clin. Chem., 49, 719–726.
Sudakov, N. P., Popkova, T. P., Novikova, M. A., Katyshev, A. V., Nikiforov, S. B., Pushkarev, B. G., Goldberg, O. A., Klimenkov, I. V., Lepekhova, S. A., Ezhikeeva, S. D., Ten, M. N., Osipov, V. G., and Konstantinov, Yu. M. (2012) The level of blood plasma mitochondrial DNA upon acute myocardium damage in experiment, Biopolym. Cel., 28, 321–324.
Pietila, K. O., Harmoinen, A. P., Jokiniitty, J., and Pasternack, A. I. (1996) Serum C-reactive protein concen-tration in acute myocardial infarction and its relationship to mortality during 24 months of follow-up in patients under thrombolytic treatment, Eur. Heart J., 17, 1345–1349.
Zorov, D. B., Juhaszova, M., and Sollott, S. J. (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release, Physiol. Rev., 94, 909–950.
Zorov, D. B., Juhaszova, M., Yaniv, Y., Nuss, H. B., Wang, S., and Sollott, S. J. (2009) Regulation and pharmacology of the mitochondrial permeability transition pore, Cardiovasc. Res., 83, 213–225.
Starkov, A. A. (2008) The role of mitochondria in reactive oxygen species metabolism and signaling, Ann. N. Y. Acad. Sci., 1147, 37–52.
Skulachev, V. P. (2006) Bioenergetic aspects of apoptosis, necrosis, and mitoptosis, Apoptosi., 11, 473–485.
Zorov, D. B., Filburn, C. R., Klotz, L. O., Zweier, J. L., and Sollott, S. J. (2000) Reactive oxygen species (ROS) induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes, J. Exp. Med., 192, 1001–1014.
Koulintchenko, M., Konstantinov, Y., and Dietrich, A. (2003) Plant mitochondria actively import DNA via the permeability transition pore complex, EMBO J., 22, 1245–1254.
Koulintchenko, M., Temperley, R. J., Mason, P. A., Dietrich, A., and Lightowlers, R. N. (2006) Natural com-petence of mammalian mitochondria allows the molecular investigation of mitochondrial gene expression, Hum. Mol. Genet., 15, 143–154.
Zorov, D. B. (1996) Mitochondrial transport of nucleic acids. Involvement of the benzodiazepine receptor, Biochemistry (Moscow), 61, 939–946.
Kaczmarek, A., Vandenabeele, P., and Krysko, D. V. (2013) Necroptosis: the release of damage-associated molecular pat-terns and its physiological relevance, Immunit., 38, 209–223.
Guescini, M., Guidolin, D., Vallorani, L., Casadei, L., Gioacchini, A. M., Tibollo, P., Battistelli, M., Falcieri, E., Battistin, L., Agnati, L. F., and Stocchi, V. (2010) C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction, Exp. Cell Res., 316, 1977–1984.
Gahan, P. B. (2012) Biology of circulating nucleic acids and possible roles in diagnosis and treatment in diabetes and cancer, Infect. Disord. Drug Target., 12, 360–370.
Zhang, Q., Itagaki, K., and Hauser, C. J. (2010) Mitochondrial DNA is released by shock and activates neu-trophils via p38 map kinase, Shoc., 34, 55–59.
Yousefi, S., Mihalache, C., Kozlowski, E., Schmid, I., and Simon, H. U. (2009) Viable neutrophils release mitochon-drial DNA to form neutrophil extracellular traps, Cell Death Differ., 16, 1438–1444.
Plotnikov, E. Y., Silachev, D. N., Jankauskas, S. S., Rokitskaya, T. I., Chupyrkina, A. A., Pevzner, I. B., Zorova, L. D., Isaev, N. K., Antonenko, Y. N., Skulachev, V. P., and Zorov, D. B. (2012) Mild uncoupling of respira-tion and phosphorylation as a mechanism providing nephron- and neuroprotective effects of penetrating cations of the SkQ family, Biochemistry (Moscow), 77, 1029–1037.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sudakov, N.P., Popkova, T.P., Katyshev, A.I. et al. Level of blood cell-free circulating mitochondrial DNA as a novel biomarker of acute myocardial ischemia. Biochemistry Moscow 80, 1387–1392 (2015). https://doi.org/10.1134/S000629791510020X
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000629791510020X