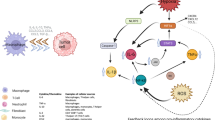
Abstract
The idea of a potential link between cancer and inflammation was first proposed by R. Virchow in the nineteenth century. However, clear evidence regarding a key role of inflammation in oncogenesis appeared only during the last decade. Now the tumor microenvironment is commonly considered as an obligatory and significant component of almost all types of cancer, and the cells infiltrating such microenvironment are a source of inflammatory cytokines. Such cytokines play a key role in regulating inflammation during both normal immune response and developing cancer. In this review, we explore the role of two inflammatory cytokines interleukin 1 and interleukin 6 in cancer development. These cytokines have pleiotropic effects on various cell types in the tumor microenvironment, particularly being able to regulate pro-oncogenic transcription factors NF-κB and STAT3. For this reason, such cytokines influence key parameters of oncogenesis, increasing cell resistance to apoptosis, proliferation of cancer cells, angiogenesis, invasion and malignancy as well as the ability of tumor cells to respond to anticancer therapy. Here we summarize novel experimental data regarding mechanisms underlying the interaction between chronic inflammation and malignant neoplasms.
Similar content being viewed by others
Abbreviations
- APC:
-
adenoma polyposis coli gene
- CAC:
-
colitis-associated cancer
- HFD:
-
high fat diet
- IL1:
-
interleukin 1
- IL6:
-
interleukin 6
- IL6R:
-
IL6 receptor
- IL1R:
-
IL1 receptor
- IL1Ra:
-
interleukin 1 receptor antagonist
- LPS:
-
lipopolysaccharide
- Th9:
-
T-helper type 9
- Th17:
-
T-helper type 17
- TLR:
-
Tolllike receptor
- TNF:
-
tumor necrosis factor
References
Mantovani, A., Romero, P., Palucka, A. K., and Marincola, F. M. (2008) Tumour immunity: effector response to tumour and role of the microenvironment, Lancet, 371, 771–783.
Balkwill, F., and Coussens, L. M. (2004) Cancer: an inflammatory link, Nature, 431, 405–406.
Coussens, L. M., and Werb, Z. (2002) Inflammation and cancer, Nature, 420, 860–867.
Galon, J., Costes, F., Sanchez-Cabo, A., Kirilovsky, A., Mlecnik, B., Lagorce-Pages, C., Tosolini, M., Camus, M., Berger, A., Wind, P., Zinzindohoue, F., Bruneval, P., Cugnenc, P. H., Trajanoski, Z., Fridman, W. H., and Pages, F. (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome, Science, 313, 1960–1964.
Kuraishy, A., Karin, M., and Grivennikov, S. I. (2011) Tumor promotion via injuryand death-induced inflammation, Immunity, 35, 467–477.
Osawa, E., Nakajima, A., Fujisawa, T., Kawamura, Y. I., Toyama-Sorimachi, N., Nakagama, H., and Dohi, T. (2006) Predominant T-helper type 2-inflammatory responses promote murine colon cancers, Int. J. Cancer, 118, 2232–2236.
Canavan, C., Abrams, K. R., and Mayberry, J. (2006) Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease, Aliment. Pharmacol. Ther., 23, 1097–1104.
Grivennikov, S. I., Greten, F. R., and Karin, M. (2010) Immunity, inflammation, and cancer, Cell, 140, 883–899.
Grivennikov, S. I. (2013) Inflammation and colorectal cancer: colitis-associated neoplasia, Semin. Immunopathol., 35, 229–244.
Grivennikov, S. I., Wang, K., Mucida, D., Stewart, C. A., Schnabl, B., Jauch, D., Taniguchi, K., Yu, G. Y., Osterreicher, C. H., Hung, K. E., Datz, C., Feng, Y., Fearon, E. R., Oukka, M., Tessarollo, L., Coppola, V., Yarovinsky, F., Cheroutre, H., Eckmann, L., Trinchieri, G., and Karin, M. (2012) Adenoma-linked barrier defects and microbial products drive IL23/IL17-mediated tumour growth, Nature, 491, 254–258.
Grivennikov, S. I., and Karin, M. (2010) Inflammation and oncogenesis: a vicious connection, Curr. Opin. Genet. Dev., 20, 65–71.
Hanahan, D., and Weinberg, R. A. (2011) Hallmarks of cancer: the next generation, Cell, 144, 646–674.
Greten, F. R., and Karin, M. (2005) NF-κB: linking inflammation and immunity to cancer development and progression, Nat. Rev. Immunol., 5, 749–759.
Natoli, G. (2009) Control of NF-kappaB-dependent transcriptional responses by chromatin organization, Cold Spring Harb. Perspect. Biol., 1, a000224.
Barish, G. D., Yu, R. T., Karunasiri, M., Ocampo, C. B., Dixon, J., Benner, C., Dent, A. L., Tangirala, R. K., and Evans, R. M. (2010) Bcl-6 and NF-kappaB cistromes mediate opposing regulation of the innate immune response, Genes Dev., 24, 2760–2765.
Smale, S. T. (2011) Hierarchies of NF-kappaB target-gene regulation, Nat. Immunol., 12, 689–694.
Atreya, R., Mudter, J., Finotto, S., Mullberg, J., Jostock, T., Wirtz, S., Schutz, M., Bartsch, B., Holtmann, M., Becker, C., Strand, D., Czaja, J., Schlaak, J. F., Lehr, H. A., Autschbach, F., Schurmann, G., Nishimoto, N., Yoshizaki, K., Ito, H., Kishimoto, T., Galle, P. R., RoseJohn, S., and Neurath, M. F. (2000) Blockade of interleukin 6 trans-signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo, Nat. Med., 6, 583–588.
Karin, M., Cao, Y., Greten, F. R., and Li, Z. W. (2002) NF-κB in cancer: from innocent bystander to major culprit, Nat. Rev. Cancer, 2, 301–310.
Karin, M., and Lin, A. (2002) NF-κB at the crossroads of life and death, Nat. Immunol., 3, 221–227.
Karin, M. (2009) NF-kappaB as a critical link between inflammation and cancer, Cold Spring Harb. Perspect. Biol., 1, a000141.
Atreya, I., Atreya, R., and Neurath, M. F. (2008) NFkappaB in inflammatory bowel disease, J. Intern. Med., 263, 591–596.
Nagasaki, T., Hara, M., Nakanishi, H., Takahashi, H., Sato, M., and Takeyama, H. (2014) Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour–stroma interaction, Br. J. Cancer, 110, 469–478.
Louis, P., Hold, G. L., and Flint, H. J. (2014) The gut microbiota, bacterial metabolites and colorectal cancer, Nat. Rev. Microbiol., 12, 661–672.
Yu, H., Pardoll, D., and Jove, R. (2009) STATs in cancer inflammation and immunity: a leading role for STAT3, Nat. Rev. Cancer, 9, 798–809.
Kishimoto, T. (2005) Interleukin-6: from basic science to medicine–40 years in immunology, Annu. Rev. Immunol., 23, 1–21.
Kishimoto, T. (1989) The biology of interleukin-6, Blood, 74, 1–10.
Neurath, M. F. (2014) Cytokines in inflammatory bowel disease, Nat. Rev. Immunol., 14, 329–342.
Calabrese, L. H., and Rose-John, S. (2014) IL-6 biology: implications for clinical targeting in rheumatic disease, Nat. Rev. Rheumatol., 10, 720–727.
Hirano, T., Ishihara, K., and Hibi, M. (2000) Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors, Oncogene, 19, 2548–2556.
Heinrich, P. C., Behrmann, I., Muller-Newen, G., Schaper, F., and Graeve, L. (1998) Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway, Biochem. J., 334, 297–314.
Wang, Y., Van Boxel-Dezaire, A. H., Cheon, H., Yang, J., and Stark, G. R. (2013) STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor, Proc. Natl. Acad. Sci. USA, 110, 16975–16980.
Yang, J., Liao, X., Agarwal, M. K., Barnes, L., Auron, P. E., and Stark, G. R. (2007) Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB, Genes Dev., 21, 1396–1408.
Vasquez-Dunddel, D., Pan, F., Zeng, Q., Gorbounov, M., Albesiano, E., Fu, J., Blosser, R. L., Tam, A. J., Bruno, T., Zhang, H., Pardoll, D., and Kim, Y. (2013) STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients, J. Clin. Invest., 123, 1580–1589.
Schaper, F., and Rose-John, S. (2015) Interleukin-6: biology, signaling and strategies of blockade, Cytokine Growth Factor Rev., 26, 475–487.
Rose-John, S. (2014) The biology of interleukin-6 in the 21st century, Semin. Immunol., 26, 1.
Becker, C., Fantini, M. C., Schramm, C., Lehr, H. A., Wirtz, S., Nikolaev, A., Burg, J., Strand, S., Kiesslich, R., Huber, S., Ito, H., Nishimoto, N., Yoshizaki, K., Kishimoto, T., Galle, P. R., Blessing, M., Rose-John, S., and Neurath, M. F. (2004) TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling, Immunity, 21, 491–501.
Matsumoto, S., Hara, T., Mitsuyama, K., Yamamoto, M., Tsuruta, O., Sata, M., Scheller, J., Rose-John, S., Kado, S., and Takada, T. (2010) Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model, J. Immunol., 184, 1543–1551.
Grivennikov, S., Karin, E., Terzic, J., Mucida, D., Yu, G. Y., Vallabhapurapu, S., Scheller, J., Rose-John, S., Cheroutre, H., Eckmann, L., and Karin, M. (2009) IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer, Cancer Cell, 15, 103–113.
Rose-John, S., Scheller, J., Elson, G., and Jones, S. A. (2006) Interleukin-6 biology is coordinated by membranebound and soluble receptors: role in inflammation and cancer, J. Leukoc. Biol., 80, 227–236.
Jones, S. A., Richards, P. J., Scheller, J., and Rose-John, S. (2005) IL-6 trans-signaling: the in vivo consequences, J. Interferon Cytokine Res., 25, 241–253.
Tawara, K., Oxford, J. T., and Jorcyk, C. L. (2011) Clinical significance of interleukin (IL)-6 in cancer metastasis to bone: potential of anti-IL-6 therapies, Cancer Manag. Res., 3, 177–189.
Chang, Q., Bournazou, E., Sansone, P., Berishaj, M., Gao, S. P., Daly, L., Wels, J., Theilen, T., Granitto, S., Zhang, X., Cotari, J., Alpaugh, M. L., De Stanchina, E., Manova, K., Li, M., Bonafe, M., Ceccarelli, C., Taffurelli, M., Santini, D., Altan-Bonnet, G., Kaplan, R., Norton, L., Nishimoto, N., Huszar, D., Lyden, D., and Bromberg, J. (2013) The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis, Neoplasia, 15, 848–862.
Guo, Y., Xu, F., Lu, T., Duan, Z., and Zhang, Z. (2012) Interleukin-6 signaling pathway in targeted therapy for cancer, Cancer Treat. Rev., 38, 904–910.
Rossi, J. F., Lu, Z. Y., Jourdan, M., and Klein, B. (2015) Interleukin-6 as a therapeutic target, Clin. Cancer Res., 21, 1248–1257.
Nguyen, D. P., Li, J., and Tewari, A. K. (2014) Inflammation and prostate cancer: the role of interleukin 6 (IL-6), BJU Int., 113, 986–992.
Baltgalvis, K. A., Berger, F. G., Pena, M. M., Davis, J. M., Muga, S. J., and Carson, J. A. (2008) Interleukin-6 and cachexia in ApcMin/+ mice, Am. J. Physiol. Regul. Integr. Comp. Physiol., 294, 393–401.
He, G., Yu, G. Y., Temkin, V., Ogata, H., Kuntzen, C., Sakurai, T., Sieghart, W., Peck-Radosavljevic, M., Leffert, H. L., and Karin, M. (2010) Hepatocyte IKKbeta/NFkappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation, Cancer Cell, 17, 286–297.
Naugler, W. E., Sakurai, T., Kim, S., Maeda, S., Kim, K., Elsharkawy, A. M., and Karin, M. (2007) Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production, Science, 317, 121–124.
He, G., Dhar, D., Nakagawa, H., Font-Burgada, J., Ogata, H., Jiang, Y., Shalapour, S., Seki, E., Yost, S. E., Jepsen, K., Frazer, K. A., Harismendy, O., Hatziapostolou, M., Iliopoulos, D., Suetsugu, A., Hoffman, R. M., Tateishi, R., Koike, K., and Karin, M. (2013) Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling, Cell, 155, 384–396.
He, G., and Karin, M. (2011) NF-kappaB and STAT3–key players in liver inflammation and cancer, Cell Res., 21, 159–168.
Calle, E. E. (2007) Obesity and cancer, BMJ, 335, 1107–1108.
Kaaks, R. (2004) Nutrition, insulin, IGF-1 metabolism and cancer risk: a summary of epidemiological evidence, Novartis Found. Symp., 262, 247–260; discussion, 260-268.
El-Serag, H. B., and Rudolph, K. L. (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis, Gastroenterology, 132, 2557–2576.
Park, E. J., Lee, J. H., Yu, G. Y., He, G., Ali, S. R., Holzer, R. G., Osterreicher, C. H., Takahashi, H., and Karin, M. (2010) Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression, Cell, 140, 197–208.
Mohamed-Ali, V., Goodrick, S., Rawesh, A., Katz, D. R., Miles, J. M., Yudkin, J. S., Klein, S., and Coppack, S. W. (1997) Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo, J. Clin. Endocrinol. Metab., 82, 4196–4200.
Fried, S. K., Bunkin, D. A., and Greenberg, A. S. (1998) Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid, J. Clin. Endocrinol. Metab., 83, 847–850.
Garlanda, C. (2013) The interleukin-1 family: back to the future, Immunity, 39, 1003–1018.
Apte, R. N., and Voronov, E. (2008) Is interleukin-1 a good or bad “guy” in tumor immunobiology and immunotherapy? Immunol. Rev., 222, 222–241.
Kawai, T., and Akira, S. (2007) TLR signaling, Semin. Immunol., 19, 24–32.
Carpenter, S., and O’Neill, L. A. (2007) How important are Toll-like receptors for antimicrobial responses? Cell Microbiol., 9, 1891–1901.
Fettelschoss, A., Kistowska, M., LeibundGut-Landmann, S., Beer, H. D., Johansen, P., Senti, G., Contassot, E., Bachmann, M. F., French, L. E., Oxenius, A., and Kundig, T. M. (2011) Inflammasome activation and IL-1beta target IL-1alpha for secretion as opposed to surface expression, Proc. Natl. Acad. Sci. USA, 108, 18055–1860.
Rider, P., Carmi, Y., Guttman, O., Braiman, A., Cohen, I., Voronov, E., White, M. R., Dinarello, C. A., and Apte, R. N. (2011) IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation, J. Immunol., 187, 4835–4843.
Gross, O., Yazdi, A. S., Thomas, C. J., Masin, M., Heinz, L. X., Guarda, G., Quadroni, M., Drexler, S. K., and Tschopp, J. (2012) Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1, Immunity, 36, 388–400.
Nakae, S., Saijo, S., Horai, R., Sudo, K., Mori, S., and Iwakura, Y. (2003) IL-17 production from activated T-cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist, Proc. Natl. Acad. Sci. USA, 100, 5986–5990.
Carmi, Y., Rinott, G., Dotan, S., Elkabets, M., Rider, P., Voronov, E., and Apte, R. N. (2011) Microenvironmentderived IL-1 and IL-17 interact in the control of lung metastasis, J. Immunol., 186, 3462–3471.
Voronov, E., Dotan, S., Krelin, Y., Song, X., Elkabets, M., Carmi, Y., Rider, P., Idan, C., Romzova, M., Kaplanov, I., and Apte, R. N. (2013) Unique versus redundant functions of IL-1alpha and IL-1beta in the tumor microenvironment, Front Immunol., 4, 177.
Carmi, Y., Dotan, S., Rider, P., Kaplanov, I., White, M. R., Baron, R., Abutbul, S., Huszar, M., Dinarello, C. A., Apte, R. N., and Voronov, E. (2013) The role of IL-1beta in the early tumor cell-induced angiogenic response, J. Immunol., 190, 3500–3509.
Voronov, E., Shouval, D. S., Krelin, Y., Cagnano, E., Benharroch, D., Iwakura, Y., Dinarello, C. A., and Apte, R. N. (2003) IL-1 is required for tumor invasiveness and angiogenesis, Proc. Natl. Acad. Sci. USA, 100, 2645–2650.
Dinarello, C. A. (2006) The paradox of pro-inflammatory cytokines in cancer, Cancer Metastasis Rev., 25, 307–313.
Dinarello, C. A. (2011) Interleukin-1 in the pathogenesis and treatment of inflammatory diseases, Blood, 117, 3720–3732.
Finnegan, A., and Doodes, P. D. (2008) Pathways for interleukin-1-driven arthritis, Arthritis Rheum., 58, 3283–3285.
Apte, R. N., Dvorkin, T., Song, X., Fima, E., Krelin, Y., Yulevitch, A., Gurfinkel, R., Werman, A., White, R. M., Argov, S., Shendler, Y., Bjorkdahl, O., Dohlsten, M., Zoller, M., Segal, S., and Voronov, E. (2000) Opposing effects of IL-1 alpha and IL-1 beta on malignancy patterns. Tumor cell-associated IL-1 alpha potentiates anti-tumor immune responses and tumor regression, whereas IL-1 beta potentiates invasiveness, Adv. Exp. Med. Biol., 479, 277–288.
Voronov, E., Carmi, Y., and Apte, R. N. (2014) The role IL-1 in tumor-mediated angiogenesis, Front. Physiol., 5, 114.
Apte, R. N., Krelin, Y., Song, X., Dotan, S., Recih, E., Elkabets, M., Carmi, Y., Dvorkin, T., White, R. M., Gayvoronsky, L., Segal, S., and Voronov, E. (2006) Effects of micro-environmentand malignant cell-derived interleukin-1 in carcinogenesis, tumour invasiveness and tumour–host interactions, Eur. J. Cancer, 42, 751–759.
Apte, R. N., Dotan, S., Elkabets, M., White, M. R., Reich, E., Carmi, Y., Song, X., Dvozkin, T., Krelin, Y., and Voronov, E. (2006) The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor–host interactions, Cancer Metastasis Rev., 25, 387–408.
Salcedo, R., Worschech, A., Cardone, M., Jones, Y., Gyulai, Z., Dai, R. M., Wang, E., Ma, W., Haines, D., O’Huigin, C., Marincola, F. M., and Trinchieri, G. (2010) MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18, J. Exp. Med., 207, 1625–1636.
Vegran, F., Berger, H., Boidot, R., Mignot, G., Bruchard, M., Dosset, M., Chalmin, F., Rebe, C., Derangere, V., Ryffel, B., Kato, M., Prevost-Blondel, A., Ghiringhelli, F., and Apetoh, L. (2014) The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells, Nat. Immunol., 15, 758–766.
Lust, J. A., Lacy, M. Q., Zeldenrust, S. R., Dispenzieri, A., Gertz, M. A., Witzig, T. E., Kumar, S., Hayman, S. R., Russell, S. J., Buadi, F. K., Geyer, S. M., Campbell, M. E., Kyle, R. A., Rajkumar, S. V., Greipp, P. R., Kline, M. P., Xiong, Y., Moon-Tasson, L. L., and Donovan, K. A. (2009) Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1 beta-induced interleukin 6 production and the myeloma proliferative component, Mayo Clin. Proc., 84, 114–122.
Lust, J. A., and Donovan, K. A. (1999) The role of interleukin-1 beta in the pathogenesis of multiple myeloma, Hematol. Oncol. Clin. North Am., 13, 1117–1125.
Tosolini, M., Kirilovsky, A., Mlecnik, B., Fredriksen, T., Mauger, S., Bindea, G., Berger, A., Bruneval, P., Fridman, W. H., Pages, F., and Galon, J. (2011) Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer, Cancer Res., 71, 1263–1271.
Bettelli, E., Carrier, Y., Gao, W., Korn, T., Strom, T. B., Oukka, M., Weiner, H. L., and Kuchroo, V. K. (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T-cells, Nature, 441, 235–238.
Mangan, P. R., Harrington, L. E., O’Quinn, D. B., Helms, W. S., Bullard, D. C., Elson, C. O., Hatton, R. D., Wahl, S. M., Schoeb, T. R., and Weaver, C. T. (2006) Transforming growth factor-beta induces development of the T(H)17 lineage, Nature, 441, 231–234.
Ivanov, I. I., McKenzie, B. S., Zhou, L., Tadokoro, C. E., Lepelley, A., Lafaille, J. J., Cua, D. J., and Littman, D. R. (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ Thelper cells, Cell, 126, 1121–1133.
Yang, X. O., Pappu, B. P., Nurieva, R., Akimzhanov, A., Kang, H. S., Chung, Y., Ma, L., Shah, B., Panopoulos, A. D., Schluns, K. S., Watowich, S. S., Tian, Q., Jetten, A. M., and Dong, C. (2008) T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma, Immunity, 28, 29–39.
Mathur, A. N., Chang, H. C., Zisoulis, D. G., Stritesky, G. L., Yu, Q., O’Malley, J. T., Kapur, R., Levy, D. E., Kansas, G. S., and Kaplan, M. H. (2007) Stat3 and Stat4 direct development of IL-17-secreting Th cells, J. Immunol., 178, 4901–4907.
Coward, J., Kulbe, H., Chakravarty, P., Leader, D. A., Vassileva, V., Leinster, D. A., Thompson, R., Schioppa, T., Nemeth, J. A., Vermeulin, J., Singh, N., Avril, N. E., Cummings, J., Rexhepaj, E., Jirstrom, K., Gallagher, W. M., Brennan, D. J., McNeish, I. A., and Balkwill, F. R. (2011) Interleukin-6 as a therapeutic target in human ovarian cancer, Clin. Cancer Res., 17, 6083–6096.
Voorhees, P. M., Manges, R. F., Sonneveld, P., Jagannath, S., Somlo, G., Krishnan, A., Lentzsch, S., Frank, R. C., Zweegman, S., Wijermans, P. W., Orlowski, R. Z., Kranenburg, B., Hall, B., Casneuf, T., Qin, X., Van De Velde, H., Xie, H., and Thomas, S. K. (2013) A phase 2 multicentre study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma, Br. J. Haematol., 161, 357–366.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O. S. Dmitrieva, I. P. Shilovskiy, M. R. Khaitov, S. I. Grivennikov, 2016, published in Biokhimiya, 2016, Vol. 81, No. 2, pp. 166–178.
Rights and permissions
About this article
Cite this article
Dmitrieva, O.S., Shilovskiy, I.P., Khaitov, M.R. et al. Interleukins 1 and 6 as main mediators of inflammation and cancer. Biochemistry Moscow 81, 80–90 (2016). https://doi.org/10.1134/S0006297916020024
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297916020024